|
from Yellowstone Science 25(1)
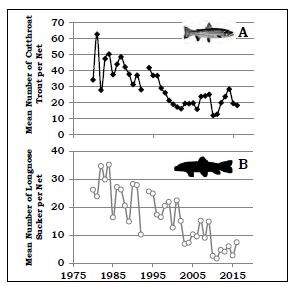
by Todd M. Koel, Jeffery L. Arnold, Lisa A. Baril, Kerry A. Gunther, Douglas W. Smith, John M. Syslo, & Lusha M. TronstadThe mountainous region within and bordering southeastern Yellowstone National Park (YNP) is among the most remote in the contiguous United States. Lying completely within wilderness, the watershed of the upper Yellowstone River is pristine. Snowmelt waters feed numerous tributaries to the Yellowstone River, which ultimately winds northward to Yellowstone Lake. The Yellowstone River contributes one-third of the flow to Yellowstone Lake within a watershed that encompasses >2,600 square kilometers (>1,004 square miles) upstream of the Great Falls at Canyon. A majority of Yellowstone Lake shoreline is undeveloped, and the lake is covered with ice for approximately five months (January-May) each year. The lake is large (35,391 hectares [87,453 acres]), deep (43 meters [141 feet] average depth; Kaplinski 1991), and mesotrophic, which means it has a moderate amount of nutrients and is productive with clear, cold, oxygen-saturated water. Active geothermal features influence water temperature and chemistry in localized areas. Following glacial recession from the region about 8,000-10,000 years ago, plant and animal species recolonized the Yellowstone Lake basin. On Two Ocean Pass, waters flowing to the Pacific and Atlantic oceans coalesce in a single stream that then splits, sending water in two different directions. Apparently, cutthroat trout originating in the Snake River drainage to the south were able to naturally cross the Continental Divide in this area, and colonize Yellowstone Lake and the river downstream (Behnke and Tomelleri 2002). Aside from the longnose dace, cutthroat trout were the only fish species evolving over several thousand years in Yellowstone Lake free of exposure to predatory fish. Park managers later stocked non-native fish species into Yellowstone Lake, including the redside shiner, lake chub, and longnose sucker. These species remain today as viable, reproducing populations. Native Food Web of Yellowstone LakeCutthroat trout evolved as an important component of a food web within Yellowstone Lake, with several resident and migratory animal species relying on them as a source of energy during critical periods of the year (figure 1). Cutthroat trout of all ages are generally found in shallow waters of the lake (less than 20 meters [66 feet] below the surface) where, during open-water seasons, they are accessible by predatory raptors and colonial water birds. Each spring, when snowmelt run-off begins to decline, spawning cutthroat trout move extensively within the lake and river system, ascending 60 or more tributary streams to Yellowstone Lake, including the expansive upper Yellowstone River system. During spawning migrations, these large-bodied, mature, and energy-rich cutthroat trout become highly vulnerable to predation in shallow streams and are consumed by grizzly and black bears, river otters, white pelicans, and other species. Overall, 4 mammal and 16 bird species feed on cutthroat trout in Yellowstone Lake and its tributaries (see “Birds and Mammals that Consume Yellowstone Cutthroat Trout,” this issue).Within Yellowstone Lake, cutthroat trout feed on macroinvertebrates (e.g., amphipods, insect larvae) and zooplankton (e.g., copepods, cladocerans; Tronstad et al. 2010). The zooplankton feed on phytoplankton, which are the free-floating, photosynthesizing algae within the lake. The larger zooplankton species are highly efficient feeders on phytoplankton, and cutthroat trout are able to filter them from the water. When cutthroat trout are abundant, densities of larger zooplankton are reduced, allowing for a relative increase in the smaller-sized species, which are incapable of feeding on the larger size range of algal particles within the phytoplankton. This increased density of phytoplankton reduces lake water clarity. Transport of Nutrients by Cutthroat TroutCutthroat trout accumulate substantial nutrients and energy in their bodies as they grow within Yellowstone Lake. During their spawning migrations, cutthroat trout physically transport these lake-derived nutrients long distances into the lake’s tributaries (Tronstad et al. 2015). Cutthroat trout enter tributaries from May to early July and spend 1-3 weeks in the streams before returning to the lake. During spawning migrations, cutthroat trout transport nutrients and energy in their carcasses (if preyed upon), deposit gametes when they spawn, and excrete ammonium and other nutrients via normal body metabolism. Nutrients and energy transported by migratory, adult cutthroat trout enhance conditions for the growth of developing fry or juvenile fish. During the period cutthroat trout are abundant within spawning tributaries, they excrete ammonium orders of magnitude higher than the background levels of ammonium naturally exported by the watershed. These nutrients, delivered by spawning cutthroat trout, enhance primary productivity by plants and algae through photosynthesis, and secondary productivity of aquatic macroinvertebrates such as mayflies, caddisflies, and midges.Introduction of Predatory Lake TroutNearly half of the waters in YNP were fishless when the park was established in 1872 because waterfalls prevented recolonization following deglaciation (Everman 1892). Early managers began seeking ways to populate these waters. In 1890, some of the first fish brought to Yellowstone were lake trout from Lake Michigan, which were stocked in Lewis and Shoshone lakes in the upper Snake River drainage (Varley 1980). Over time, the lake trout dispersed downstream, invading Heart and Jackson lakes and establishing sizable populations. Lake trout were present in Yellowstone for more than a century before they were found in Yellowstone Lake, where one was caught by an angler in 1994 (Kaeding et al. 1996). By analyzing the microchemistry of bone from several larger lake trout from Yellowstone Lake, scientists determined they had come from Lewis Lake, perhaps introduced illegally by someone (Munro et al. 2005).The detection of lake trout in Yellowstone Lake prompted the National Park Service (NPS) to initiate gillnetting. Gillnetting effort and the biomass of lake trout removed increased annually, but was not sufficient to suppress the population (Koel et al. 2005). Estimated abundance of age-2 and older lake trout increased from 125,000 fish in 1998 (Ruzycki et al. 2003) to 790,000 fish in 2012, despite the removal of over 800,000 fish during this period (Syslo et al. 2011, Syslo 2015). The NPS suppression netting effort was then greatly increased, resulting in more than 1.5 million lake trout removed from 2012 to 2016. Lake trout abundance remained >700,000 fish in 2015. Impacts of Lake Trout on Cutthroat Trout and Longnose SuckersDuring the early stages of lake trout expansion, lake trout consumption of cutthroat trout was high. The estimated 125,000 lake trout present in 1998 likely consumed 3-4 million cutthroat trout that year (Ruzycki et al. 2003, Syslo et al. 2016). Subsequent lake trout population growth and expansion resulted in a precipitous, lake-wide decline in cutthroat trout (Koel et al. 2005; figure 2). In 1987, about the time lake trout were introduced, nearly 50 cutthroat trout were caught per unit of netting effort during annual monitoring on Yellowstone Lake (figure 2). Cutthroat trout of all lengths were well represented in the population, including a high proportion of juvenile fish. The catch of cutthroat trout declined to just 13 per effort unit in 2010. The size structure of the cutthroat trout population also changed, and the proportion of juvenile fish in the population was very low.Concurrent with the decline in cutthroat trout was a steady, long-term decline in the introduced longnose sucker population within Yellowstone Lake (figure 2). Unclear, however, is the mechanism causing this decline. Longnose suckers occur, primarily, throughout the shallow-water, littoral zones of the lake, and spawn along the lake shore and in tributaries during the spring. Although analysis of diets conducted during the summer did not suggest significant predation upon suckers by lake trout in Yellowstone Lake (Ruzycki et al. 2003, Syslo et al. 2016), in Lake Tahoe suckers were the major food item of large lake trout sampled throughout the year (Franz and Cordone 1970). It is possible consumption of suckers by lake trout is higher during winter when water temperatures are extremely cold, allowing lake trout to exploit shallow water habitats where the suckers reside. Cascading Impacts on Plankton, Macroinvertebrates, and Nitrogen CyclingThe introduction of lake trout added a fourth predatory trophic level and resulted in cascading interactions within the food web of Yellowstone Lake (Carpenter et al. 1985, Spencer et al. 1991, Ellis et al. 2010). Before the decline, cutthroat trout consumed mostly larger-bodied cladocerans (Syslo et al. 2016); and as a result, smaller- bodied copepods were more prevalent within the lake (Tronstad et al. 2010; figure 1). Cladocerans represented 80% of the cutthroat trout diet in 1989, but only 11% in 2011 (Syslo et al. 2016). After the population declined, the remaining cutthroat trout consumed mostly amphipods, which represented 8% of the cutthroat trout diet in 1989, increasing to 79% in 2011 (Wilmot et al. 2016). The result was a concurrent shift within the lake’s zooplankton community from dominance by (smaller-bodied) copepods before lake trout introduction to dominance by (larger-bodied) cladocerans after lake trout introduction. Total zooplankton biomass and average length of zooplankton individuals increased after the invasion of lake trout (Tronstad et al. 2010).These changes to the zooplankton community, in turn, affected the phytoplankton community. Chlorophyll a, a concentration which is an indicator of phytoplanton biomass, was twice as high in 1972 (Knight 1975) prior to lake trout introduction than it was in 2004 and 2005 (Tronstad et al. 2010). Also, the number of phytoplankton in a given volume of water was three times higher in 1972 and 1996, and 6.5 times higher in 1997, compared with 2004. Thus, lake trout introduction, cutthroat trout decline, and a shift in the zooplankton community to larger-bodied cladocerans caused a reduction in phytoplankton abundance. This lowered abundance resulted in an increase in overall water clarity throughout this period. Secchi depths, an indicator of water clarity, averaged 1.6 m (5.2 ft.) deeper in 2006 than in 1976, prior to lake trout introduction (Tronstad et al. 2010). There were strong interactions among trophic levels within Yellowstone Lake as the effects of lake trout predation were transmitted down the food web. These impacts also extended into connected tributary streams. The lake trout-induced decline in cutthroat trout resulted in fewer spawning fish returning to tributary streams and, as a result, a significant reduction in the transport of nutrients (e.g., ammonium) from Yellowstone Lake into the tributaries (Tronstad et al. 2015). In fact, lake trout had a larger effect on nitrogen cycling within adjacent tributaries than within the lake itself because the spawning behavior of cutthroat trout concentrated them in tributaries, thus increasing the effect (Tronstad et al. 2015). This reduction in nutrients and energy flow to tributaries may have contributed to a decline in the overall productivity of those waters. Cascading Impacts on Bears and OttersThe introduction and expansion of lake trout caused significant, cascading effects that extended to land-dwelling animals, such as grizzly and black bears because spawning cutthroat trout are an important, high energy food for them within the Yellowstone Lake basin (Mattson and Reinhart 1995, Gunther et al. 2014; figure 1). The densities of cutthroat trout were high in the tributaries during spring, making them attractive to fishing by bears (Reinhart and Mattson 1990). During 1985-1987, bear activity occurred on 93% of the lake’s spawning tributaries, with evidence of fish consumption on 61% of the tributaries (Reinhart and Mattson 1990). Evidence of bear activity on spawning streams was noted 50 times on 11 frontcountry streams in 1991 when spawning cutthroat trout were abundant (Reinhart 1990, Koel et al. 2005; figure 3). However, concurrent with the cutthroat trout decline through the 1990s, evidence of bear activity also declined. No bear activity was found on any of these spawning streams in 2008, 2009, or 2011 (figure 3).In the late-1990s and after cutthroat trout had declined considerably, an estimated 14-21% of grizzly bears in the Greater Yellowstone Ecosystem (GYE) were feeding on spawning cutthroat trout in Yellowstone Lake tributaries (Haroldson et al. 2005). When compared to estimates obtained in 1997-2000, the number of grizzly bears visiting spawning streams a decade later (2007-2009) decreased by 63%, and the number of black bears decreased 64-84% (Teisberg et al. 2013). Fortin et al. (2013) estimated the biomass of cutthroat trout consumed by grizzly and black bears declined 70% and 90%, respectively, in the years between 1997- 2000 and 2007-2009. The low densities of the remaining trout were no longer efficiently fed upon by grizzly or black bears. Overall, the estimated number of spawning cutthroat trout consumed by grizzly bears annually declined from 20,910 in the late 1980s (Stapp and Hayward 2002) to 2,266 in the late 1990s (Felicetti et al. 2004) to only 302 in the late 2000s (Fortin et al. 2013). However, grizzly and black bears are opportunistic feeders with a flexible diet (Gunther et al. 2014); consequently, they made use of other foods available in the Yellowstone Lake area when cutthroat trout abundance was low (Fortin et al. 2013). Recently, evidence suggests bears are beginning to return to spawning streams to prey upon cutthroat trout. Visual surveys from 2012 to 2016 documented a slight increase in spawning cutthroat trout and bears returning to feed upon them. Evidence of bears was found during 30% of visits to the frontcountry tributaries surveyed in 2015 (figure 3). River otters are another common semi-aquatic predator that is heavily dependent upon cutthroat trout. Although otters move freely and frequently throughout Yellowstone Lake and connected streams (Crait et al. 2015), they are relatively restricted in their abilities to travel long distances over land and across drainage divides. Thus, they are thought to be vulnerable to impacts of the cutthroat trout decline. Otters followed the movements of spawning cutthroat trout and were active on spawning streams during 2002-2003. Cutthroat trout occurred in 72% of otter scat collected at 87 otter latrine sites during this period (Crait and Ben-David 2006). The otters transported lake-derived nutrients to terrestrial latrine sites, and the nutrients influenced the prevalence and growth of plants in localized areas around Yellowstone Lake (Crait and Ben-David 2007). Temporal changes in otter latrine activity occurred in response to declines in spawning cutthroat trout. By 2006-2008, otter activity at latrine sites decreased; and the prevalence of cutthroat trout in otter scat declined to 53% (Crait et al. 2015). Otters supplemented their diet with alternative prey, including non-native longnose suckers and amphibians, which are not likely comparable replacement foods. Estimates of otter abundance do not exist prior to the 2000s and before cutthroat trout began to decline; however, the estimate of 1 otter per 13.4 kilometers of shoreline along Yellowstone Lake in 2008 (Crait et al. 2015) will be used as a baseline to document changes following the recovery of cutthroat trout. Cascading Impacts on Eagles, Ospreys, and Colonial ShorebirdsYellowstone Lake supports an abundant diversity of bird life (Smith et al. 2015), including numerous fish-eating birds such as bald eagles and ospreys (McEneaney 2002). During the 1960s and 1970s, a period when pesticides impacted bald eagles, there were typically 4-6 eagle nests on Yellowstone Lake (McEneaney 2002). The number of nests increased to 16 from 1987 to 2003, but then declined to 12 nests by 2007 (figure 4). There was a steady, long-term decline in eagle nest productivity over two decades (1987-2007), concurrent with the lakewide decline in cutthroat trout. In the 1980s, 60%-80% of eagle nests on Yellowstone Lake successfully fledged young (figure 4); however, nest success declined to zero in 2009 when cutthroat trout abundance was low. Bald eagles are opportunistic feeders, and they increased consumption of alternative prey and scavenged carcasses for food. As a result, the number of bald eagle nests increased and nesting success increased to 64%-76% during 2013-2015 (Smith et al. 2015; figure 4).Yellowstone Lake once had the highest densities of nesting ospreys in YNP, but the number of osprey nests and nest productivity has dramatically changed. The number of nests declined from 60 in 2001 to 3-5 during 2008-2015 (figure 4), likely due to the decline in small cutthroat trout less than 280 mm (11 in.) in length, the preferred-size prey of ospreys (McEneaney 2002, Baril et al. 2013). Nesting success prior to 1994 was typically 49%-77%, but it declined to zero during 2008-2011 when no young ospreys were fledged from Yellowstone Lake nests (Baril et al. 2013). Only 1-2 nests have successfully fledged young in recent years (figure 4). Although a few osprey nests remain on Yellowstone Lake, these ospreys are not observed foraging for cutthroat trout. In 2010, despite more than 60 hours of active nest monitoring, no ospreys were observed foraging or attempting to forage at Yellowstone Lake (Soyland 2010). Ospreys are obligate fish eaters that do not switch to other alternative food sources in the absence of fish. The ospreys have been leaving the Yellowstone Lake area to forage in nearby waters where prey fish are more abundant, likely at Heart, Lewis, and Shoshone lakes (Soyland 2010). The number of pelicans, cormorants, gulls, and terns fledged from the Molly Islands’ colonies has been highly variable, but has declined overall since the 1990s (McEneaney 2002, Smith et al. 2015). Although the loss of cutthroat trout is thought to be a factor impacting the colonial birds, nesting success of these species is also strongly influenced by environmental factors, including air temperature, duration of ice cover, and lake surface levels (Diem and Pugesek 1994). During years with high lake water levels, for example, nests of colonial birds on the Molly Islands have flooded resulting in complete, or near complete, reproductive failures (Diem and Pugesek 1994, McEneaney 2002). In 2014, a year with a relatively early ice melt and lower than average lake surface levels, 307 pelican nests were observed on the Molly Islands, producing 276 young (Smith et al. 2015). A total of 56 nesting cormorants were also observed, which fledged an estimated 25 young. In the same year, none of the observed 18 gull nests produced any young. Long-term declining trends in colonial birds suggest a factor other than weather and lake levels is impacting them on Yellowstone Lake. For example, although as many as 28 tern nesting pairs produced 28 young on the Molly Islands in 1990, only 3 nesting pairs produced 3 young in 2001 (McEneaney 2002); and no terns have nested on the Molly Islands since 2005 (Smith et al. 2015). Hypothesized Links to Elk, Loons, & SwansThe lake trout-induced decline in cutthroat trout directly affected and displaced several avian and terrestrial consumers, but indirect effects on alternative prey are less understood. Following the cutthroat trout decline within spawning tributaries, grizzly and black bears fed less upon them and shifted their diet to other foods in the lake area, including elk calves (Fortin et al. 2013; figure 1). Each spring, thousands of elk that winter on lands at lower elevations in the GYE migrate to the interior of YNP. Elk calves born in the Yellowstone Lake area are vulnerable to predation, especially during the first few weeks after birth. From 2007 to 2009, elk accounted for 84% of all ungulates consumed by bears (Fortin et al. 2013), suggesting lake trout had an indirect impact on migratory elk (Middleton et al. 2013). Even though there is strong evidence bears were consuming fewer cutthroat trout, it is unknown whether individual bears increased predation on elk calves specifically due to the cutthroat trout decline.As cutthroat trout declined and gradually became less available, bald eagles increasingly consumed alternative foods, including scavenging of elk, bison, and other carcasses when available. Bald eagles have also been observed more frequently in recent years preying on sensitive waterfowl, including common loons and trumpeter swans (Smith et al. 2015). The south arms of Yellowstone Lake and nearby Riddle Lake are among the highest quality and highest producing loon nesting habitats in Wyoming. However, common loon nesting pairs have declined by 50% since 1990 in YNP (Evers et al. 2013). Trumpeter swans are also a sensitive species that has experienced a decline. In recent years, only two breeding pairs and 6-10 non-breeding swans spend the summer in the park. Reasons for the declines in common loons and trumpeter swans are unclear, but may include the reduced availability of cutthroat trout as a food source for loons and increased predation on loon chicks and trumpeter swan cygnets due to consumption by bald eagles in the Yellowstone Lake area. Yellowstone Lake Restoration PotentialYellowstone Lake and connecting streams and rivers lie within the heart of YNP and, as such, are among the most pristine waters remaining on Earth. The watershed of Yellowstone Lake remains largely unaltered by humans; as a result, the entire assemblage of native plant and animal species remains. Contributing to the decline of cutthroat trout in the late 1990s and 2000s was the introduction of the exotic parasite Myxobolus cerebralis, which caused whirling disease in cutthroat trout in localized areas of the ecosystem, including Pelican Creek and the Yellowstone River downstream of Yellowstone Lake through Hayden Valley (Koel et al. 2006, Alexander et al. 2011, Murcia et al. 2014). Drought, which occurred over several years in the early 2000s, resulted in low lake levels and the loss of surface water connections with many tributary streams, potentially limiting the ability of cutthroat trout fry emigration to Yellowstone Lake prior to winter (Koel et al. 2005). Although M. cerebralis has been present for nearly two decades and fish-eating birds have the ability to move the parasite throughout the ecosystem (Koel et al. 2010), whirling disease has not spread or widely influenced recruitment of cutthroat trout across Yellowstone Lake (Koel et al. 2015).The primary deleterious factor influencing the ecology of Yellowstone Lake is the presence of lake trout. There are no other well-established, introduced species or altered watersheds. Thus, it is reasonable to assume that if lake trout are suppressed and predation pressure on cutthroat trout is reduced, thereby allowing the cutthroat population to rebound, the trophic cascade we have described in this article can be largely reversed. Already, cutthroat trout have shown signs of recovery. Concurrent with a massive surge in lake trout suppression during 2012-2016, the cutthroat trout population has increased in abundance and is once again comprised of a large proportion of juvenile fish (Koel et al. 2015). Spawning adult cutthroat trout are slowly returning to several of the smaller tributaries, and bear use of these streams has increased as a result. The status of the plankton communities will be reassessed in 2017, as they would be additional, leading indicators of ecosystem change. Although lake trout abundance throughout Yellowstone Lake remains high, it’s apparent that suppression is having a positive effect. The potential for restoration of Yellowstone Lake is extremely high if lake trout suppression is maintained. ConclusionBecause of the migratory behavior of cutthroat trout, impacts of lake trout on the ecology of the Yellowstone Lake ecosystem extend far beyond the shoreline and smaller tributaries and into the extremely remote, largely unmonitored reaches of the upper Yellowstone River drainage in the Bridger-Teton Wilderness of Wyoming (Ertel 2011). Anecdotal evidence from anglers and outfitters in this region suggest large-scale declines in spawning cutthroat trout occurred during the 1990s and 2000s. However, due to remoteness and inaccessibility during much of the spring and summer spawning period, no quantitative information exists on impacts to cutthroat trout consumer species throughout this region. Because cutthroat trout are the only fish in this large drainage and most of the adults return to Yellowstone Lake immediately after spawning (Ertel 2011), it is likely that bears, otters, eagles, ospreys, and other species have been widely displaced throughout the upper Yellowstone River drainage.Herein we have documented impacts to multiple aquatic and terrestrial trophic levels across a large, complex ecosystem, free from any confounding effects of land use or other anthropogenic disturbance. Because there are no other large interconnecting lakes within this ecosystem, the lake trout have and will remain confined to Yellowstone Lake, where suppression activities are focused. However, because technologies do not exist to completely extirpate lake trout, cutthroat trout may not be able to fully recover within Yellowstone Lake and tributary spawning streams. We predict the operations to suppress lake trout will reduce their abundance, thereby allowing cutthroat trout recovery to a level where they regain their ecological importance, and again underpin and support the natural processes and biodiversity for which Yellowstone National Park is widely recognized. Literature CitedAlexander, J.D., B.L. Kerans, T.M. Koel, and C. Rasmussen. 2011. Context-specific parasitism in Tubifex tubifex in geothermally influenced stream reaches in Yellowstone National Park. Journal of the North American Benthological Society 30:853-867.Baril, L.M., D.W. Smith, T. Drummer, and T.M. Koel. 2013. Implications of cutthroat trout declines for breeding ospreys and bald eagles at Yellowstone Lake. Journal of Raptor Research 47:234-245. Behnke, R.J., and J.R. Tomelleri. 2002. Trout and salmon of North America. Free Press, New York, New York, USA. Carpenter, S.R., J.F. Kitchell, and J.R. Hodgson. 1985. Cascading trophic interactions and lake productivity. Bioscience 35:634- 639. Crait, J.R., and M. Ben-David. 2006. River otters in Yellowstone Lake depend on a declining cutthroat trout population. Journal of Mammalogy 87:485-494. Crait, J.R., and M. Ben-David. 2007. Effects of river otter activity on terrestrial plants in trophically altered Yellowstone Lake. Ecology 88:1040-1052. Crait, J.R., E.V. Regehr, and M. Ben-David. 2015. Indirect effects of bioinvasions in Yellowstone Lake: response of river otters to declines in native cutthroat trout. Biological Conservation 191:596-605. Diem, K.L., and B.H. Pugesek. 1994. American white pelicans at the Molly Islands, in Yellowstone National Park: twenty-two years of boom and bust breeding, 1966-1987. Colonial Waterbirds 17:130-145. Ellis, B.K., J.A. Stanford, D. Goodman, C.P. Stafford, D.L. Gustafson, D.A. Beauchamp, D.W. Chess, J.A. Craft, M.A. Deleray, and B.S. Hansen. 2010. Long-term effects of a trophic cascade in a large lake ecosystem. Proceedings of the National Academy of Sciences 108:1070-1075. Ertel, B.D. 2011. Distribution, movements, and life-history characteristics of Yellowstone cutthroat trout Oncorhynchus clarkii bouvieri in the upper Yellowstone River drainage. Thesis. Montana State University, Bozeman, Montana, USA. Everman, B.W. 1892. Report on the establishment of fish culture stations in the Rocky Mountain region and Gulf States. U.S. Government Printing Office, Washington, D.C., USA. Evers, D.C., V. Spagnuolo, and K. Taylor. 2013. Restore the call: Wyoming status report for the common loon. Science Communications Series BRI 2013-21. Biodiversity Research Institute, Gorham, Maine, USA. Felicetti, L.A., C.C. Schwartz, R.O. Rye, K.A. Gunther, J.G. Crock, M.A. Haroldson, L. Waits, and C.T. Robbins. 2004. Use of naturally occurring mercury to determine the importance of cutthroat trout to Yellowstone grizzly bears. Canadian Journal of Zoology 82:493-501. Fortin, J.K., C.C. Schwartz, K.A. Gunther, J.E. Teisberg, M.A. Haroldson, M.A. Evans, and C.T. Robbins. 2013. Dietary adjustability of grizzly bears and American black bears in Yellowstone National Park. Journal of Wildlife Management 77:270- 281. Frantz, T.C., and A.J. Cordone. 1970. Food of lake trout in Lake Tahoe. California Fish and Game 56:21-35. Gunther, K.A., R.R. Shoemaker, K.L. Frey, M.A. Haroldson, S.L. Cain, F.T. van Manen, and J.K. Fortin. 2014. Dietary breadth of grizzly bears in the Greater Yellowstone Ecosystem. Ursus 25:60-72. Haroldson, M.A., K.A. Gunther, D.P. Reinhart, S.R. Prodruzny, C. Cegelski, L. Waits, T. Wyman, and J. Smith. 2005. Changing numbers of spawning cutthroat trout in tributary streams of Yellowstone Lake and estimates of grizzly bears visiting streams from DNA. Ursus 16:167-180. Kaeding, L.R., G.D. Boltz, and D.G. Carty. 1996. Lake trout discovery in Yellowstone Lake threaten native cutthroat trout. Fisheries 21:16-20. Kaplinski, M.A. 1991. Geomorphology and geology of Yellowstone Lake, Yellowstone National Park, Wyoming. Thesis. Northern Arizona University, Flagstaff, Arizona, USA. Knight, J.C. 1975. The limnology of the West Thumb of Yellowstone Lake, Yellowstone National Park, Wyoming. Thesis. Montana State University, Bozeman, Montana, USA. Koel, T.M., P. Bigelow, P.D. Doepke, B.D. Ertel, and D.L. Mahony. 2005. Nonnative lake trout result in Yellowstone cutthroat trout decline and impacts to bears and anglers. Fisheries 30:10-19. Koel, T.M., D.L. Mahony, K.L. Kinnan, C. Rasmussen, C.J. Hudson, S. Murcia, and B.L. Kerans. 2006. Myxobolus cerebralis in native cutthroat trout of the Yellowstone Lake ecosystem. Journal of Aquatic Animal Health 18:157-175. Koel, T.M., B.L. Kerans, S.C. Barras, K.C. Hanson, and J.S. Wood. 2010. Avian piscivores as vectors for Myxobolus cerebralis in the Greater Yellowstone Ecosystem. Transactions of the American Fisheries Society 139:976-988. Koel, T.M., J.L. Arnold, P.E. Bigelow, C.R. Detjens, P.D. Doepke, B.D. Ertel, and M.E. Ruhl. 2015. Native fish conservation program, Yellowstone Fisheries & Aquatic Sciences 2012-2014, Yellowstone National Park. YCR-2015-01. National Park Service, Yellowstone Center for Resources, Yellowstone National Park, Wyoming, USA. Mattson, D.J., and D.P. Reinhart. 1995. Influences of cutthroat trout (Oncorhynchus clarki) on behavior and reproduction of Yellowstone grizzly bears (Ursus arctos), 1975-1989. Canadian Journal of Zoology 73:2072-2079. McEneaney, T. 2002. Piscivorous birds of Yellowstone Lake: their history, ecology, and status. Pages 121-134 in R.J. Anderson and D. Harmon, editors. Yellowstone Lake: hotbed of chaos or reservoir of resilience? Proceedings of the 6th Biennial Scientific Conference on the Greater Yellowstone Ecosystem. Yellowstone Center for Resources, Yellowstone National Park, Wyoming, USA. Middleton, A.D., T.A. Morrison, J.K. Fortin, C.T. Robbins, K.M. Proffitt, P.J. White, D.E. McWhirter, T.M. Koel, D.G. Brimeyer, W.S. Fairbanks, and M.J. Kauffman. 2013. Grizzly bear predation links the loss of native trout to the demography of migratory elk in Yellowstone. Proceedings of the Royal Society B 280:1-8. Munro, A.R., T.E. McMahon, and J.R. Ruzycki. 2005. Natural chemical markers identify source and date of introduction of an exotic species: lake trout (Salvelinus namaycush) in Yellowstone Lake. Canadian Journal of Fisheries and Aquatic Sciences 62:79-87. Murcia, S., B.L. Kerans, T.M. Koel, and E. MacConnell. 2014. Myxobolus cerebralis (Hofer) infection risk in native cutthroat trout Oncorhynchus clarkii (Richardson) and its relationships to tributary environments in the Yellowstone Lake basin. Journal of Fish Diseases 38:637-652. Reinhart, D.P. 1990. Grizzly bear use of cutthroat trout spawning streams in tributaries of Yellowstone Lake. Thesis. Montana State University, Bozeman, Montana, USA. Reinhart, D.P., and D.J. Mattson. 1990. Bear use of cutthroat trout spawning streams in Yellowstone National Park. Pages 343-350 in L.M. Darling and W.R. Archibald, editors. Bears— their biology and management: Proceedings of the Eighth International Conference on Bear Research and Management. International Association for Bear Research and Management, Madison, Wisconsin, USA. Ruzycki, J.R., D.A. Beauchamp, and D.L. Yule. 2003. Effects of introduced lake trout on native cutthroat trout in Yellowstone Lake. Ecological Applications 13:23-37. Smith, D.W., L. Baril, L. Strait, D. Haines, B. Cassidy, and K. Duffy. 2015. Yellowstone bird program 2014 annual report. YCR-2015-03. National Park Service, Yellowstone Center for Resources, Yellowstone National Park, Wyoming, USA. Soyland, A. 2010. Effects of the introduced lake trout (Salvelinus namaycush) on the osprey (Pandion haliaetus) population in Yellowstone National Park. Thesis. Institute of Nature Management, Norwegian University of Life Science, Sørhellinga, Norway. Spencer, C.N., B.R. McClelland, and J.A. Stanford. 1991. Shrimp stocking, salmon collapse, and eagle displacement. Bioscience 41:14-21. Stapp, P., and G.D. Hayward. 2002. Effects of an introduced piscivore on native trout: insights from a demographic model. Biological Invasions 4:299-316. Syslo, J.M., C.S. Guy, P.E. Bigelow, P.D. Doepke, B.D. Ertel, and T.M. Koel. 2011. Response of non-native lake trout (Salvelinus namaycush) to 15 years of harvest in Yellowstone Lake, Yellowstone National Park. Canadian Journal of Fisheries and Aquatic Sciences 68:2132-2145. Syslo, J.M. 2015. Dynamics of Yellowstone cutthroat trout and lake trout in the Yellowstone Lake ecosystem: a case study for the ecology and management of non-native fishes. Dissertation. Department of Ecology, Montana State University, Bozeman, Montana, USA. Syslo, J.M., C.S. Guy, and T.M. Koel. 2016. Feeding ecology of native and nonnative salmonids during the expansion of a nonnative apex predator in Yellowstone Lake, Yellowstone National Park. Transactions of the American Fisheries Society 145:476-492. Teisberg, J.E., M.A. Haroldson, C.C. Schwartz, K.A. Gunther, J.K. Fortin, and C.T. Robbins. 2013. Contrasting past and current number of bears visiting Yellowstone cutthroat trout streams. Journal of Wildlife Management 78:369-378. Tronstad, L.M., R.O. Hall, T.M. Koel, and K.G. Gerow. 2010. Introduced lake trout produced a four-level trophic cascade in Yellowstone Lake. Transactions of the American Fisheries Society 139:1536-1550. Tronstad, L.M., R.O. Hall, and T.M. Koel. 2015. Introduced lake trout alter nitrogen cycling beyond Yellowstone Lake. Ecosphere 6:1-24. Varley, J.D. 1980. A history of fish stocking activities in Yellowstone National Park between 1881 and 1980. Information paper 35. U.S. Department of the Interior, National Park Service, Yellowstone National Park, Wyoming, USA. Wilmot, O., L. Tronstad, R.O. Hall, T. Koel, and J. Arnold. 2016. Lake trout-induced spatial variation in the benthic invertebrates of Yellowstone Lake. Park Science 32:25-35. 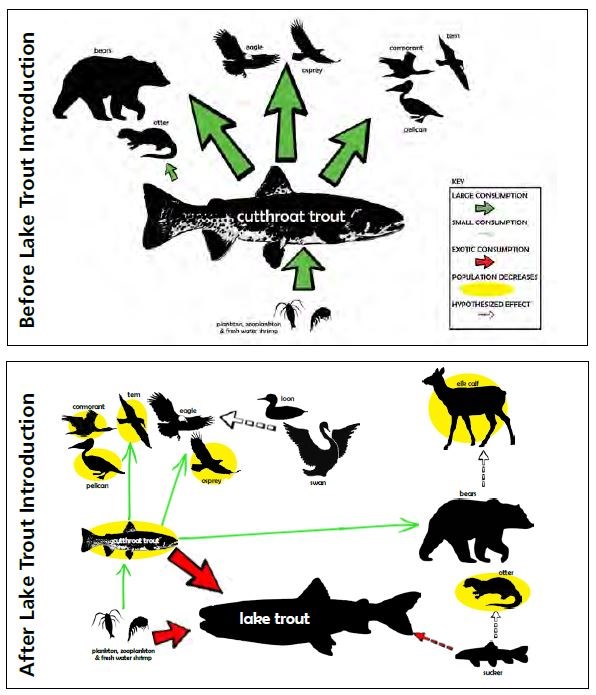
(approximately 100 mm 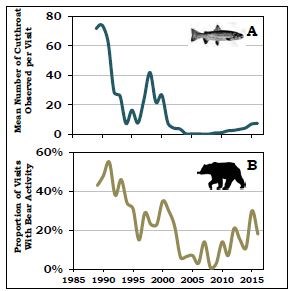
the western side of Yellowstone Lake between Lake and Grant, 1989-2016. 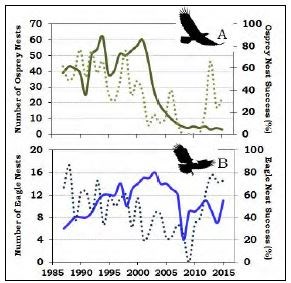
|
Last updated: February 16, 2017
