Piscicides are fish toxins approved by the Environmental Protection Agency and used by managers to eradicate non-native fishes. With the exception of sea lamprey control in the Great Lakes, all fish removal projects in the United States utilize piscicide formulations containing either rotenone or antimycin as the active ingredient. Both of these natural compounds have been used extensively in fisheries management since the 1930s to control invasive species, recover native species, or restore sport fish (e.g., removing suckers to make habitat available for a sport fish). Over the past decade, biologists in Yellowstone National Park (YNP) have used rotenone in High Lake and East Fork Specimen Creek (2006-2009), Goose Lake (2011), Elk Creek (2012-2014), Grayling Creek (2013-2014), and Soda Butte Creek (2015-2016) to remove non-native fish species. Piscicides are effective at removing fish from habitats where nets, electrofishing, angling, traps, or other mechanical methods are impractical or ineffective. However, piscicides are also non-specific, meaning they can impact all gill-breathing organisms, including larval amphibians and macroinvertebrates. Therefore, when using this powerful tool, natural resource managers need to consider these potential impacts and seek ways to lessen them.
What is Rotenone, and How Does it Work?
Rotenone occurs in the roots, stems, and leaves of tropical plants in the pea family (Fabaceae), including the jewel vine (Derris involuta), lancepod or cube plant (Lonchocarpus utilis), and Tephrosia genus found in southeast Asia, South America, and east Africa, respectively. Rotenone and other related compounds are produced by these plants for a variety of functions, including defense against the growth of microorganisms (Dixon and Passinetti 2010). Indigenous peoples discovered that the roots of these plants are toxic to fish and developed a variety of ways to apply these roots to water to kill fish for consumption (Cannon et al. 2004).
Rotenone’s toxicity results from the inhibition of a biochemical reaction called oxidative phosphorylation, which occurs in the energy-producing mitochondria within the cells of animals. The resulting loss of usable energy for cellular function results in death. To reach most tissues in an animal, rotenone must first be absorbed into the bloodstream. Ingestion of rotenone has a relatively minor effect on land animals because the enzymes and acids of the digestive system break it down, thus limiting absorption through the lining of the intestinal tract. On the other hand, the absorption of rotenone in water across the gill membrane by fish or other aquatic organisms (amphibians, immature insects) is a direct route into the blood.
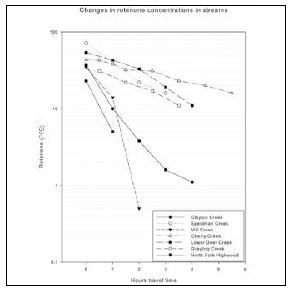
The physical and chemical properties of rotenone combine with environmental conditions to determine its fate and toxicity in the environment. It is a large compound quickly broken down in the environment by sunlight and other factors. The degradation time in lakes ranges from one day in warm water to several weeks in cold water. Rotenone is also somewhat hydrophobic, meaning once applied in the environment, it will readily bind to sediments or organic matter in the water. These factors result in a relatively quick dissipation and degradation of rotenone from the environment, which poses a challenge for biologists attempting to kill fish before rotenone concentrations decrease to nontoxic levels. This challenge is greatest in rapidly moving waters where rotenone, once applied, will either degrade or bind to streambed sediments within 1-5 hours of travel time, thus requiring reapplication to maintain toxic concentrations (figure 1).
Impacts of Rotenone on Organisms Other than Fish
The use of rotenone to kill fish can affect non-target organisms. In YNP, this includes most gill-breathing, immature forms of aquatic macroinvertebrates, specifically the insect taxa Ephemeroptera (mayflies), Plecoptera (stoneflies), and Tricoptera (caddisflies; Magnum and Madrigal 1999, Hamilton et al. 2009). Factors such as age and size contribute to sensitivity, as younger insects have thinner cuticles and smaller animals have higher surface area to volume ratios, both leading to greater absorption of rotenone. Life history characteristics are also a factor, since animals living on the water-sediment interface of lakes or streams are more likely to be exposed to rotenone than those living in the spaces between gravel/cobble or burrowed into mud (Minckley and Mihilack 1981, Whelan 2002). Additionally, insects with high oxygen requirements will typically succumb more quickly to rotenone because it inhibits the oxygen-mediated production of energy molecules in the body (Engstrom-Heg et al. 1978). Finally, aquatic invertebrates with tracheal gills for respiration generally are more sensitive to rotenone than those that acquire oxygen through the skin, air, or respiratory pigments (Vinson et al. 2010).
Immature gill-breathing forms of amphibians may also be inadvertently impacted by rotenone treatments. In YNP, this potentially includes the boreal toad (Anaxyrus boreas), a species of concern in YNP and the Rocky Mountain West, blotched tiger salamander (Ambystoma tigrinum melanostictum), boreal chorus frog (Pseudacris maculata), and Columbia spotted frog (Rana luteiventris). Rotenone generally has a greater impact on larval forms of both frogs and salamanders than on adult forms (Farringer 1972, Burress 1982, Fontenot et al. 1994, Grisak et al. 2007). However, during the larval stage, frogs undergo lung development as they approach metamorphosis and rely very little on gill respiration; whereas, toads remain gill-breathers during the entire larval period (McDiarmid and Altig 1999). Frog tadpoles, therefore, may be less susceptible to the negative effects of rotenone as they grow older.
Research Conducted During East Fork Specimen Creek Rotenone Treatments
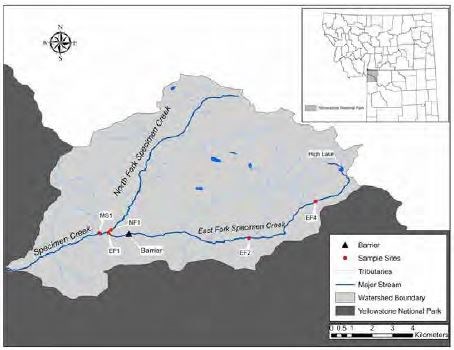
Rotenone treatments were used in the East Fork of Specimen Creek (EFSC) drainage during 2006-2009 to remove non-native trout. The first treatment occurred in 2006 at the upper end of the drainage, which included High Lake and its outlet stream, ending at a waterfall barrier (Koel et al. 2008). This was followed by treatment of Specimen Creek in 2008 and 2009 from the waterfall to a man-made barrier near the confluence with the North Fork Specimen Creek (figure 2), approximately 27 km (16.6 mi.) downstream. In all treatments, a liquid formulation of rotenone called CFT-Legumine (5% active rotenone) was applied at a concentration of 1 part per million (ppm). For the stream treatments, the primary means of applying rotenone was through the use of “drip stations” consisting of a 5-gallon container filled with a CFT-Legumine/water mixture, metered out at a constant rate to maintain a 1 ppm concentration in the stream. Because the rotenone degraded and bound with streambed materials after it was applied, there was a decrease in the concentration of rotenone in the water with increasing distance from each drip station. Therefore, it was necessary to utilize additional drip stations spaced evenly throughout the treated section. On Specimen Creek, each drip station applied rotenone for 8 hours; stations were spaced at a distance equivalent to 2-3 hours travel time in the stream water.
The CFT-Legumine rotenone formulation used at High Lake and the EFSC was a relatively new product whose impacts to non-target organisms had not been previously evaluated in the field. In spite of this information gap, this formulation was chosen over traditional products because it contained oil-based solvents, which contain fewer and less persistent contaminants, and reduced odor, rather than petroleum-based solvents. Biologists used this opportunity to investigate how CFT-Legumine impacts benthic macroinvertebrates (EFSC) and amphibians (High Lake and outlet EFSC) in an effort to identify ways to mitigate effects during future treatments.
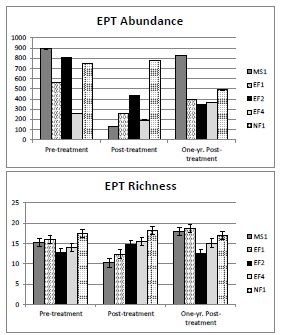
Benthic Macroinvertebrates – Scientists measured changes in the abundance and richness (number of taxa) of macroinvertebrates at a total of five sites: two sites on the EFSC downstream of rotenone drip stations, two sites below the detoxification station where potassium permanganate was applied to deactivate rotenone (see “Westslope Cutthroat Trout and Fluvial Arctic Grayling Restoration,” this issue), and one reference (non-treated) site (Skorupski 2011; figure 3). Sampling occurred at each site before the rotenone treatments, immediately after treatments, and one year after treatments. A total of 57 insect taxa were found at the five sampling sites, dominated by the true flies (family Chironomidae), mayflies, stoneflies, and caddisflies. The CFT-Legumine treatment slightly reduced the abundance and richness of mayflies, stoneflies, and caddisflies immediately following the treatment, but did not impact overall insect richness. Macroinvertebrates sampled within the detoxification area experienced similar, but greater, effects from the potassium permanganate than individuals within the treatment area that were exposed to rotenone. However, the abundance and richness of mayflies, stoneflies, and caddisflies returned to pre-treatment levels at most sites within a year (figure 3).
Five taxa found during pre-treatment sampling were not detected after the rotenone treatments. There were also six taxa not detected before treatment that were collected after treatment. None of these taxa are endangered or listed as sensitive by the Montana Natural Heritage Program (2010). Skorupski (2011) indicated these “missing” taxa were probably an artifact of low abundance because most were not consistently detected during all the years (2004-2010) of previous sampling in the drainage. Also, the amount of streambed sampled during treatments was small (less than one square meter at each site), which could contribute to rare taxa going undetected.
The phenomenon of missing taxa is not uncommon in macroinvertebrate sampling. A study was conducted on the Logan River in Utah in which the river was sampled for macroinvertebrates once a month for 10 years. The monthly samples routinely counted an average of 28 different genera. However, the cumulative number of taxa continued to rise over the 10-year period to 84 genera, demonstrating the difficulty in developing a complete inventory of all taxa in a waterbody (Vinson et al. 2010). Overall, the best way to characterize impacts of rotenone to macroinvertebrate communities is to use metrics such as total abundance, total taxa, or community indices.
Critics of piscicide treatments have used missing taxa as an argument against the use of these chemicals, claiming it shows they will extirpate sensitive taxa from treatment areas. Treatments of the Green River in Wyoming and the Strawberry River in Utah are often cited because some taxa disappeared after rotenone was applied (Binns 1967, Magnum and Madrigal 1999). However, those treatments were conducted using much higher concentrations and older, petroleum-based formulations of rotenone for longer periods of time than used currently in YNP.
Skorupski (2011) also evaluated downstream drift by benthic macroinvertebrates during rotenone treatments. Drift is a behavior used by benthic macroinvertebrates to disperse to new areas in search of food or habitat, as well as a response to stressors such as fire, floods, and, in this case, rotenone (Waters 1972). On EFSC, taxa differed in their drift response to rotenone. Stoneflies were the first to drift, showing a marked increase in drift after only 30-60 minutes of exposure. This was followed by other non-insect species, the true flies, and then mayflies, which showed peak drift at 180-210 minutes after exposure. The slowest taxa to respond were caddisflies and beetles, with low drift rates that were still increasing after 330-360 minutes. Although these results suggest some macroinvertebrates can avoid rotenone by drifting downstream and out of the treatment area, a better understanding of the ultimate fate of drifting insects would be useful for interpreting the significance of species not detected during sampling before and after rotenone treatments.
Amphibian Tadpoles – Another study examined the effects of rotenone on the distribution and abundance of Columbia spotted frog tadpoles in High Lake and nearby wetlands before (2006) and after (2007-2009) the rotenone treatment (Billman et al. 2012; figure 4). Scientists measured impacts of the rotenone treatment on different life stages, as well as the impacts of Yellowstone cutthroat trout and westslope cutthroat trout in High Lake on the frogs (Billman et al. 2011, 2012). The Gosner (1960) staging system for amphibian development was used to assign a number to the stages of larval development through complete metamorphosis, with stage 1 being the fertilized egg and stage 46 being the full adult form. Frog tadpoles begin to develop lungs and rely less on gills for respiration during the late stages (40-45).
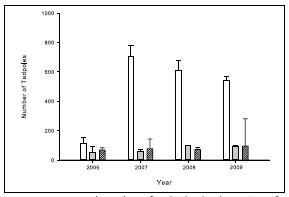
Prior to the rotenone treatment, tadpoles (stages 40- 43) and adult spotted frogs were placed in mesh sentinel cages at different locations in the treatment area and in two of the nearby wetlands not being treated with rotenone (figure 4). While the caged animals in the nearby wetlands survived, all tadpoles in the cages held in High Lake died as a result of the treatment. All adult spotted frogs held in High Lake survived. No live tadpoles were found elsewhere in the lake after the treatment, but nongill- breathing juveniles and adults were found at multiple locations (Billman et al. 2012).
Immediately prior to the rotenone treatment in 2006, tadpole abundance in High Lake was similar to that found in the much smaller nearby wetlands (figure 4). Over the next three years, tadpole abundance in the wetlands was essentially unchanged. In 2007, the first year following treatment, tadpole abundance in High Lake increased about sixfold, but then declined slightly in each of 2008 and 2009. The marked increase in tadpoles following the rotenone treatment was likely a result of the absence of trout in High Lake, as the rotenone treatment removed over 800 trout from the lake, some as large as 400 mm in length (Koel et al. 2007). These fish likely preyed on the tadpoles and suppressed their numbers. The estimate of the tadpole population in 2007 was made before more than 8,300 westslope cutthroat trout were reintroduced into the lake and inlet streams between 2007 and 2009 (Koel et al. 2011). Therefore the slight downward trend in tadpole abundance from 2007 to 2009 likely reflects the impact of reintroduced trout on the tadpoles. Observations of tadpole behavior support this hypothesis. In 2006 when fish were present, tadpoles were only found in the sedge-protected portions of the outlet channel to the lake, while adults were found throughout the lake. When fish were absent in 2007, tadpoles were found throughout the outlet and in margins around the main portion of the lake. By 2009, tadpoles were again restricted to sedge-protected portions of the outlet margins (Billman et al. 2012).
Laboratory experiments were also conducted in 2008 and 2009 to determine the toxicity of rotenone to different life stages of Columbia spotted frog and boreal toad tadpoles (Billman et al. 2011). After a 96-hour exposure to CFT-Legumine at levels ranging from 0.1-1.0 ppm, older Columbia spotted frog tadpoles (stages 40- 45) were found to be less susceptible to rotenone than younger tadpoles (stages 21-25 or 30-35). The youngest Columbia spotted frog tadpoles had 100% mortality at concentrations of 0.5 and 1.0 ppm, but only 1% mortality at 0.1 ppm. Medium-aged tadpoles (stages 30-35) had between 73-100% mortality at 1.0 ppm, but only 2% at lower concentrations. At 1 ppm, the oldest Columbia spotted frog tadpoles experienced 57% mortality in 2008 but only 6% mortality in 2009. Conversely, boreal toad tadpoles experienced high mortality at 1 ppm across age groups (83%-99%; Billman et al. 2011).
The reduced mortality of older tadpoles was likely due to their increased reliance on lungs for respiration rather than on gills. Gill surfaces are the quickest route for an organism to absorb rotenone, so it is not surprising that the laboratory studies found gill-breathers are more sensitive than lung-breathers. These findings are also consistent with observations of frogs in High Lake following the treatment in 2006, where non gill-breathing juveniles were found alive, while no gill-breathing larvae survived the treatment.
Summary
This work provides strong evidence that rotenone treatments have not significantly impacted benthic macroinvertebrates or amphibians in YNP in the longterm. Rotenone degraded quickly within streams, and many macroinvertebrates escaped the treatment area via downstream drift. Sampling of macroinvertebrates within stream substrates indicated a slight reduction in the abundance of mayflies, stoneflies, and caddisflies immediately following rotenone treatments. However, abundance increased to pre-treatment levels within one year. This variation was most pronounced at sites located immediately downstream of detoxification stations, suggesting that the potassium permanganate, a strong oxidizing agent also used to purify city drinking water, may have a slightly greater effect on macroinvertebrates than the CFT-Legumine rotenone formulation itself.
Both Skorupski (2011) and Billman et al. (2011) recommended using a minimum dosage of rotenone to reduce potential impacts on non-target organisms. All High Lake and EFSC treatments were conducted using a CFT-Legumine concentration of 1.0 ppm, and the drip stations on the creek applied rotenone for 8 hours. Skorupski (2011) noted that if the duration of stream treatments could be reduced from 8 to 4 hours, then the amount of macroinvertebrate drift would be reduced by roughly 50%, which would probably be less harmful to the macroinvertebrates. Billman et al. (2011) suggested treating at a lower dose, if possible, based on laboratory experiments which revealed that the percentage of early and middle stage tadpoles that died after 96 hours of exposure to 0.5 ppm was less than half the percentage that died when exposed to 1.0 ppm. These recommendations are consistent with the CFT-Legumine label guidance to treat at a concentration of 0.5-1.0 ppm for “normal” types of use, for a duration of 4-8 hours in streams. Following the EFSC treatment, YNP biologists have generally treated streams for 4 hours. Bioassays are also conducted prior to any treatment (see “Westslope Cutthroat Trout and Fluvial Arctic Grayling Restoration,” this issue) to determine the minimum dose necessary to effectively kill fish (Finlayson et al. 2010).
Studies on EFSC also provided guidance on how to appropriately time, or sequence, treatments to reduce impacts to non-target species. Skorupski (2011) suggested partitioning the drainage into multiple treatments zones with intermediate barriers, and leaving time between treatments to allow for dispersal and recolonization of invertebrates from untreated areas. He also recommended not treating headwater areas that are fishless, which would then leave a source for recolonization of downstream treated reaches. Billman et al. (2011, 2012) noted impacts to amphibians could be reduced if treatments were timed to occur when tadpoles were no longer present or were in their older life stages. All treatments in YNP have and will continue to be conducted during late summer or fall to avoid impacts on amphibians.
Literature Cited
Billman, H.G, S. St-Hilaire, C.G. Kruse, T.S. Peterson, and C.R. Peterson. 2011. Effects of rotenone on Columbia spotted frog and boreal toad tadpoles. Transactions of American Fisheries Society 140:919-927.
Billman, H.G, C.G. Kruse, S. St-Hilaire, T.M. Koel, J.L. Arnold, and C.R Peterson. 2012. Effects of rotenone on Columbia spotted frog Rana luteiventris during field application in lentic habitats in southwestern Montana. North American Journal of Fisheries Management 32:781-789.
Binns, N.A. 1967. Effects of rotenone treatment on the fauna of the Green River, Wyoming. Wyoming Game and Fish Commission. Cheyenne, Wyoming, USA.
Burress, R.M. 1982. Effects of synergized rotenone on nontarget organisms in ponds. Investigations in Fish Control 91. U.S. Fish and Wildlife Service, Washington, D.C., USA.
Cannon, J.G., R.A. Burton, S.G. Wood, and N.L. Owen. 2004. Naturally occurring fish poisons from plants. Journal of Chemical Education 81:1457-1461.
Dixon, R.A., and G.M. Pasinetti. 2010. Flavonoids and isoflavonoids: from plant biology to agriculture and neuroscience. Plant Physiology 154:453-457.
Engstrom-Heg, R., R. Colesante, and E. Silco. 1978. Rotenone tolerances of stream bottom insects. New York Fish and Game Journal 25: 31-41.
Farringer, J.E. 1972. The determination of the aquatic toxicity of rotenone and Bayer 73 to selected aquatic organisms. Thesis. University of Wisconsin-Lacrosse, Lacrosse, Wisconsin, USA.
Finlayson, B., R. Schnick, D. Skaar, J. Anderson, L. Demong, D. Duffield, W. Horton, and J. Steinkjer. 2010. Planning and operating procedures for the use of rotenone in fish management – rotenone SOP manual. American Fisheries Society, Bethesda, Maryland, USA.
Fontenot, L.W., G.P. Noblet, and S.G. Platt. 1994. Rotenone hazards to amphibians and reptiles. Herpetological Review 25:150-156.
Gosner, K.L. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183-190.
Grisak, G.G., D.R. Skaar, G.L. Michael, M.E. Schnee and B.L. Marotz. 2007. Toxicity of Fintrol (antimycin) and Prenfish (rotenone) to three amphibian species. Intermountain Journal of Sciences 13:1-8.
Hamilton, B.T., S.E. Moore, T.B. Williams, N. Darby, and M.R. Vinson. 2009. Comparative effects of rotenone and antimycin on benthic macroinvertebrate diversity in two streams in Great Basin National Park. North American Journal of Fisheries Management 29:1620-1635.
Koel, T.M., J.L. Arnold, P.E. Bigelow, P.D. Doepke, B.D. Ertel, and M.E. Ruhl. 2007. Yellowstone Fisheries & Aquatic Sciences: annual report, 2006. YCR-2007-04. National Park Service, Yellowstone Center for Resources, Yellowstone National Park, Wyoming, USA.
Koel, T.M., J.L. Arnold, P.E. Bigelow, P.D. Doepke, B.D. Ertel, and M.E. Ruhl. 2008. Yellowstone Fisheries & Aquatic Sciences: annual report, 2007. YCR-2008-02. National Park Service, Yellowstone National Park, Wyoming, USA.
Koel, T.M., J.L. Arnold, P.E. Bigelow, P.D. Doepke, B.D. Ertel, and M.E. Ruhl. 2011. Yellowstone Fisheries & Aquatic Sciences: annual report, 2009–2010. YCR-2011-11. National Park Service, Yellowstone Center for Resources, Yellowstone National Park, Wyoming, USA.
Montana Natural Heritage Program. 2010. Animal species of concern database. http://mtnhp.org/docs/2010_Animal_SOC. pdf
McDiarmid, R.W., and R. Altig. 1999. Tadpoles: the biology of anuran larvae. University of Chicago Press, Chicago, Illinois, USA.
Magnum, F.A., and J.L. Madrigal. 1999. Rotenone effects on aquatic macroinvertebrates of the Strawberry River, Utah: a five year summary. Journal of Freshwater Ecology 14:125-135.
Minkley, W.L., and P. Mihalick. 1981. Effects of chemical treatment for fish eradication on stream-dwelling invertebrates. Journal of the Arizona-Nevada Academy of Sciences 16:79-82.
Skorupski, J.A. 2011. Effects of CFT-Legumine rotenone on macroinvertebrates in four drainages in Montana and New Mexico. Thesis. University of North Texas, Denton, Texas, USA.
Vinson, M.R., E.C. Dinger, and D.K. Vinson. 2010. Piscicides and invertebrates: after 70 years, does anyone really know? Fisheries 35:61-71.
Waters, T.F. 1972. The drift of stream insects. Annual Review of Entomology 17:253-272.
Whelan, J. 2002. Aquatic macroinvertebrate monitoring results of the 1995 and 1996 rotenone treatments of Manning Creek, Utah. Publication No. 02-02. Utah Department of Natural Resources, Salt Lake City, Utah, USA.
Don Skaar is Special Projects Bureau Chief for the Fisheries Division of Montana Fish, Wildlife & Parks in Helena. His experience applying piscicides spans more than 30 years, and he has been an instructor of the American Fisheries Society course “Planning and Executing Successful Rotenone and Antimycin Projects” since 2007.