Part of a series of articles titled Park Air Profiles.
Article
Park Air Profiles - Isle Royale National Park
Air Quality at Isle Royale National Park
Most visitors expect clean air and clear views in parks. Isle Royale National Park (NP), Michigan, is a heavily forested, remote island in Lake Superior that experiences relatively good air quality. However, air pollution from mainland sources in Canada and the Midwest, including pollutants from industries along the Ohio River Valley, does affect the park. Air pollutants blown into the park can harm natural and scenic resources such as soils, surface waters, plants, wildlife, and visibility. The National Park Service works to address air pollution effects at Isle Royale NP, and in parks across the U.S., through science, policy and planning, and by doing our part.
Nitrogen and Sulfur

Nitrogen (N) and sulfur (S) compounds deposited from the air may have harmful effects on ecosystem processes. Healthy ecosystems can naturally buffer a certain amount of pollution, but once a threshold is passed the ecosystem may respond negatively. This threshold is the critical load, or the amount of pollution above which harmful changes in sensitive ecosystems occur (Porter 2005). N and S deposition change ecosystems through eutrophication (N deposition) and acidification (N + S deposition). Eutrophication increases soil and water nutrients which causes some species to grow more quickly and changes community composition. Ecosystem sensitivity to nutrient N enrichment at Isle Royale National Park (ISRO) relative to other national parks is very low (Sullivan et al. 2016); for a full list of N sensitive ecosystem components, see: NPS ARD 2019. Acidification leaches important cations from soils, lakes, ponds, and streams which decreases habitat quality. Ecosystem sensitivity to acidification at ISRO relative to other national parks is high (Sullivan et al. 2016); to search for acid-sensitive plant species, see: NPSpecies.
From 2017-2019 total N deposition in ISRO ranged from 4.0 to 6.5 kg-N ha-1 yr-1 and total S deposition ranged from 1.6 to 2.4 kg-S ha-1 yr-1 based on the TDep model (NADP, 2018). See the conditions and trends website for park-specific information on N and S deposition at ISRO.
Boreal lakes—including Sargent and Richie—may be particularly sensitive to N enrichment, which could rapidly affect algal communities and lake biodiversity (Saros 2008; Sullivan et al. 2016).
At ISRO, thin, undeveloped soils, and low buffering capacity result in surface waterways and soils that are vulnerable to acidification (Sullivan et al. 2016). S is a concern at ISRO because it plays an essential role in the methylation of mercury, leading to toxic accumulation of methylmercury in fish and wildlife.
Epiphytic macrolichen community responses
Epiphytic macrolichens grow on tree trunks, branches, and boles. Since these lichens grow above the ground, they obtain all their nutrients directly from precipitation and the air. Many epiphytic lichen species have narrow environmental niches and are extremely sensitive to changes in air pollution. Geiser et al. (2019) used a U.S. Forest Service national survey to develop critical loads of nitrogen (N) and critical loads of sulfur (S) to prevent more than a 20% decline in four lichen community metrics: total species richness, pollution sensitive species richness, forage lichen abundance, and cyanolichen abundance.
McCoy et al. (2021) used forested area from the National Land Cover Database to estimate the impact of air pollution on epiphytic lichen communities. Forested area makes up 380 km2 (17.1%) of the land area of Isle Royale National Park.
- N deposition exceeded the 3.1 kg-N ha-1 yr-1 critical load to protect N-sensitive lichen species richness in 100% of the forested area.
- S deposition was below the 2.7 kg-S ha-1 yr-1 critical load to protect S-sensitive lichen species richness in every part of the forested area.
For exceedances of other lichen metrics and the predicted decline of lichen communities see Appendices A and B of McCoy et al. (2021).
Additional modeling was done on 459 lichen species to test the combined effects of air pollution and climate gradients (Geiser et al. 2021). A critical load indicative of initial shifts from pollution-sensitive toward pollution-tolerant species occurred at 1.5 kg-N ha-1 yr-1 and 2.7 kg-S ha-1 yr-1 even under changing climate regimes.
Plant species response
Plants vary in their tolerance of eutrophication and acidification, and some plant species respond to nitrogen (N) or sulfur (S) pollution with declines in growth, survival, or abundance on the landscape. Horn et al. (2018) used the U.S. Forest Service national forest survey to develop critical loads of N and critical loads of S to prevent declines in growth or survival of sensitive tree species. Clark et al. (2019) used a database of plant community surveys to develop critical loads of N and critical loads of S to prevent a decline in abundance of sensitive herbaceous plant species. According to NPSpecies, Isle Royale National Park contains:
- 2 N-sensitive tree species and 68 N-sensitive herbaceous species.
- 6 S-sensitive tree species and 55 S-sensitive herbaceous species.
Mycorrhizal fungi community response
Many plants have a symbiotic relationship with mycorrhizal fungi (MF). Through the roots, the plants supply the fungi with carbon from photosynthesis and in exchange the MF enhance nutrient availability within soils, increase drought tolerance, and provide physical resistance to soil erosion (George et al., 1995; Cheng et al., 2021; Burri et al., 2013). Anthropogenic Nitrogen (N) deposition can disrupt this symbiotic relationship resulting in a shift from N sensitive to N tolerant mycorrhizal fungi and plant communities.
With increased N deposition to the soil, MF become less important for nutrient uptake and many plants will cease the exchange of nutrients altogether making them more vulnerable to stressors such as drought (Lilleskov et al., 2019). The CL-N for the shift in mycorrhizal community is 5-6 kg-N ha-1 yr-1 in coniferous forests and 10-20 kg-N ha-1 yr-1 broadleaf forests.
Isle Royale National Park has 42.4 km2 of coniferous forests, 54.4 km2 of broadleaf forests, and 419.7 km2 of mixed forests. Using the range in critical loads above, the minimum CL is exceeded in 11.4% of forested area and the maximum CL is exceeded in 0% of forested area based on 2019-2021 TDep Total N deposition.
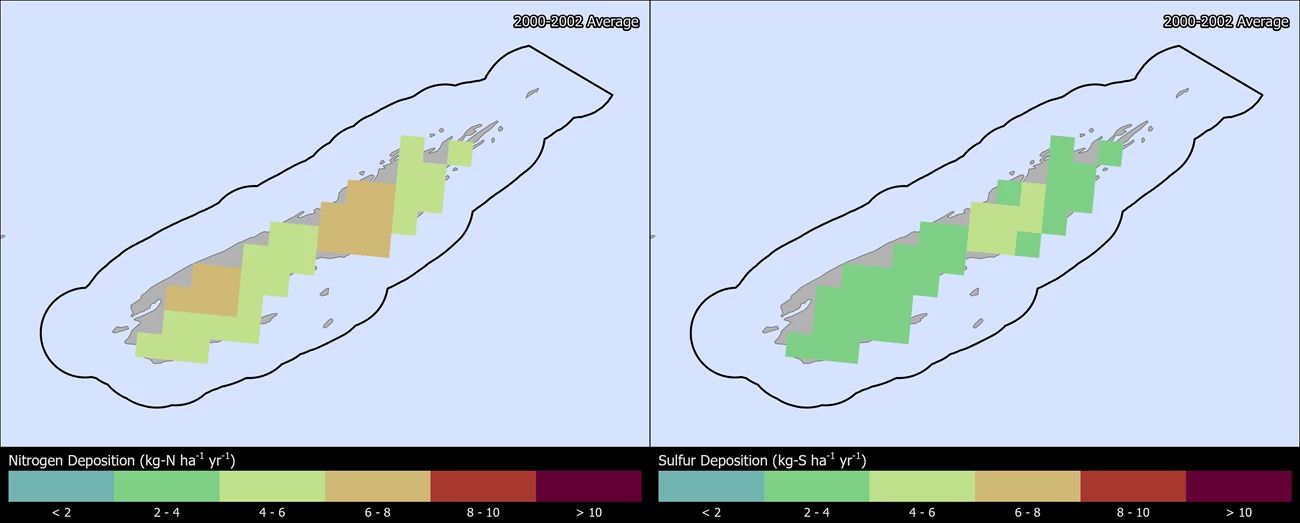
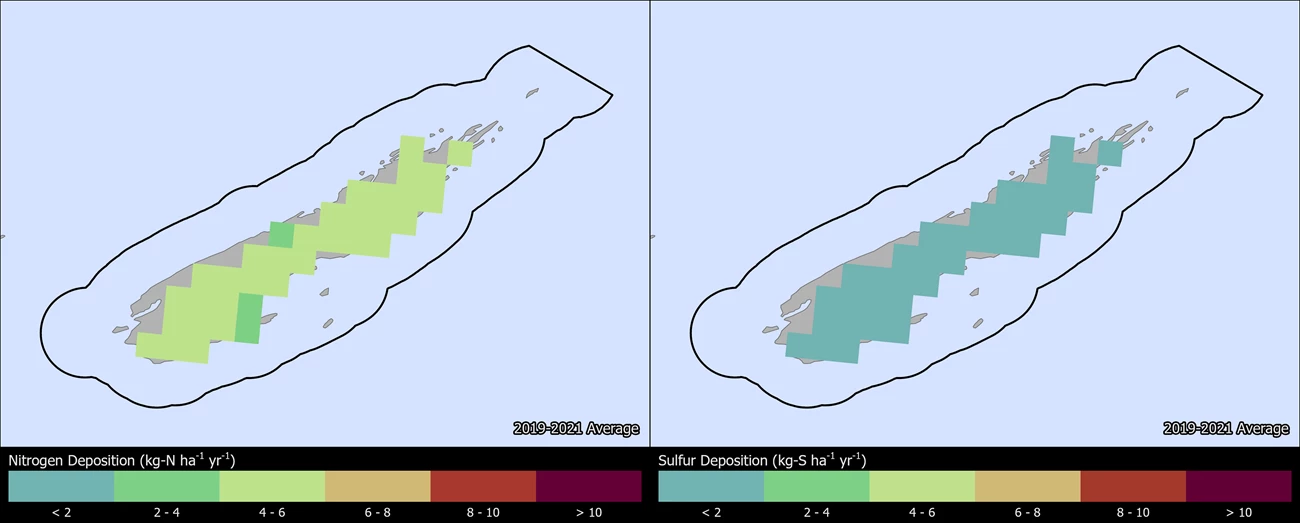
Change in N and S deposition from 2000 to 2021
The maps below show how the spatial distribution of estimated Total N and Total S deposition in ISRO has changed from 2000-2002 to 2019-2021 (TDep MMF version 2022.02). Slide the arrows in the middle of the image up and down to compare N and S deposition between the two years (Yearly Data).
- Minimum N deposition decreased from 4.9 to 4.0 kg-N ha-1 yr-1 and maximum N deposition decreased from 7.7 to 5.9 kg-N ha-1 yr-1.
- Minimum S deposition decreased from 3.3 to 0.9 kg-S ha-1 yr-1 and maximum S deposition decreased from 4.0 to 1.2 kg-S ha-1 yr-1.
Persistent Pollutants
Pollutants like mercury and pesticides are concerning because they are persistent and toxic in the environment. These contaminants can travel in the air thousands of miles away from the source of pollution, even depositing in protected places like national parks. In addition, while some of these harmful pollutants may be banned from use, historically contaminated sites continue to endure negative environmental consequences.
When deposited, airborne mercury and other toxic air contaminants are known to harm wildlife like birds and fish, and cause human health concerns. Many of these substances enter the food chain and accumulate in the tissue of organisms causing reduced reproductive success, impaired growth and development, and decreased survival.Isle Royale NP is particularly sensitive to mercury pollution. The abundance of wetlands, low pH lakes, complex food webs, and predatory fish creates an environment susceptible to the bioaccumulation of toxics. Power plants and other sources of air pollution on the mainland contribute to the deposition of toxics at Isle Royale NP.
- Mercury concentrations in some fish sampled at Isle Royale NP exceeded the threshold for human consumption. Preliminary data from five sites in the park indicate an average fish mercury concentration of 0.246 ppm ww. Mercury concentrations in 30% of fish sampled (n=162) exceeded the US EPA threshold established for human consumption (0.3 ppm ww) (Eagles-Smith et al. 2019). However, the data may not reflect the risk at other unsampled locations in the park. Fish consumption advisories may be in effect for mercury and other contaminants (NPS 2022).
- Some dragonfly larvae sampled at Isle Royale NP had mercury concentrations at moderate or higher impairment levels. Dragonfly larvae have been sampled and analyzed for mercury from five sites in the park; 58% of the data fall into the moderate (100-300 ng/g dw) and 8% fall into the high (300-700 ng/g dw) impairment categories for potential mercury risk. An index of moderate impairment or higher suggests some fish may exceed the US EPA benchmark for protection of human health (Eagles-Smith et al. 2018; Eagles-Smith et al. 2020). Haro et al. 2013 similarly detected mercury in dragonfly larvae from the park and identified a positive correlation with mercury concentrations in fish.
- Mercury in wet deposition in the Great Lakes region has been monitored by various long-term studies since the 1990’s (Evers et al. 2011a; Weiner et al. 2011; Weiss-Penzias et al. 2016). The data show that declining trends in mercury deposition generally occurred until 2008, after which relatively flat or even positive trends occurred. Elevated levels of mercury were found in rain and snow at monitoring sites near Voyageurs NP (Risch et al. 2012).
- Contaminants and pesticides were found in park rainfall and surface water. A study in four Midwestern U.S. national parks found more chemicals and higher concentrations were found at parks with greater urban influences than at the more remote parks. Atrazine and DEET were detected in park surface water samples (Elliott and VanderMeulen 2017). Contaminants were also found in elevated concentrations in tributaries to the Great Lakes (Elliot et al. 2017; Thomas et al. 2017).
- Over three decades of studies continue to show elevated concentrations of many contaminants—specifically mercury and PCBs—in park air, vegetation, precipitation, sediment, fish, mussels, loons, gull eggs, mice, and moose teeth (Bowerman et al. 2011; Cain et al. 2011; Chernyk et al. 2002; Drevnick et al. 2008; Evers et al. 2011a; Evers et al. 2011b; Evers et al. 1998; Gorski et al. 2003; Hermanson and Hites 1990; NPS 2010; Route 2011; Sandheinrich and Drevnick 2016; Sandheinrich et al. 2011; Scheuhammer and Blancher 1994; Swackhamer and Hornbuckle 2004; Thurman and Cromwell 2000; Vucetich et al. 2009; Vucetich et al. 2001; Wiener et al. 2022; Wiener et al. 2016; Wiener et al. 2012; Wiener et al. 2006).
The NPS Air Resources Division reports on park conditions and trends for mercury. Visit the webpage to learn more.
Visibility

Visitors come to Isle Royale NP to enjoy the spectacular remote islands in the vastness of Lake Superior, with forests, inland lakes, and opportunities to see wildlife. Park vistas are sometimes obscured by haze, reducing how well and how far people can see. Visibility reducing haze is caused by tiny particles in the air, and these particles can also affect human health. Many of the same pollutants that ultimately fall out as nitrogen and sulfur deposition contribute to this haze. Additionally, organic compounds, soot, and dust reduce visibility. Smoke from nearby forest fires also contributes to particulate matter in the region. Significant improvements in park visibility have been documented since the 2000’s. Overall, visibility in the park still needs improvement to reach the Clean Air Act goal of no human caused impairment.
Visibility effects:
- Reduced visibility, at times, due to human-caused haze and fine particles of air pollution, including dust;
- Reduction of the average natural visual range from about 115 miles (without pollution) to about 100 miles because of pollution at the park;
- Reduction of the visual range to below 45 miles on very hazy days.
Visit the NPS air quality conditions and trends website for park-specific visibility information. Isle Royale NP has been monitoring visibility since 1988. Explore scenic vistas of Lake Superior and other sites in the Great Lakes via live webcams, and explore air monitoring »
Ground-Level Ozone

At ground level, ozone is harmful to human health and the environment. Ground-level ozone does not come directly from smokestacks or vehicles, but instead is formed when other pollutants, mainly nitrogen oxides and volatile organic compounds, react in the presence of sunlight. Isle Royal National Park may be affected by high levels of ozone formation over the Great Lakes (Stroud et al. 2020, Pierce et al. 2023)
Over the course of a growing season, ozone can damage plant tissues making it harder for plants to grow and store carbon. Ozone causes leaf injuries like bleaching or dark spots on some sensitive plants. A park risk assessment concluded that plants in Isle Royale NP were at low risk of injury to leaves (Kohut 2004). There are 10 plants that may display ozone injury at Isle Royale National Park. Search ozone-sensitive plant species found at Isle Royale National Park.
US Environmental Protection Agency and NPS found in ozone exposure experiments that ozone slowed tree seedling growth. NPS uses W126 values from averaged seedling responses in those experiments to describe park condition in terms of Vegetation Health. Ozone affects actively growing plants, so the W126 metric weights a sum of ozone concentrations during daylight hours over three months in the growing season.
A recent re-analysis of the seedling experiments established critical levels of ozone protective of each tree species tested (Lee et al. 2022). The ozone critical levels are W126 values that will prevent 5% or greater deficit in tree seedling biomass. Air Quality Conditions and Trends reports a 5-year average of W126 for each park. In 2018-2022, the average W126 value for Isle Royale National Park was 3.9 ppm-h. Based on this ozone level, trees present in the park (NPSpecies) are at low risk of ozone effects:
-
Tree species red maple (Acer rubrum), sugar maple (Acer saccharum), quaking aspen (Populus tremuloides) and eastern white pine (Pinus strobus) are at low risk from ozone despite their known sensitivity. Recent ozone levels in the park are below critical levels that protect these trees from 5% biomass deficit.
Ozone critical levels are for tree seedlings, which represent the regenerative capacity and long-term stability of sensitive species within a forest. These tree species are also known to be sensitive to ozone as adults (Bell et al. 2020), but critical values for seedling growth do not predict ozone effects on mature trees. Air Resources Division is currently working with collaborators to establish critical levels for mature trees using data from forest monitoring plots.
Visit the NPS air quality conditions and trends website for park-specific ozone information.
Explore Other Park Air Profiles
There are 47 other Park Air Profiles covering parks across the United States and its territories.
References
Bell MD, Felker-Quinn E, Kohut R. 2020. Ozone sensitive plant species on National Park Service lands. Natural Resource Report. NPS/WASO/NRR—2020/2062. National Park Service. Fort Collins, Colorado. https://irma.nps.gov/DataStore/Reference/Profile/2271702
Bowerman, W., Moore, L., Leith, K., Drouillard, K., Sikarskie, J., Best, D., Allan, T., Garvon, J., Scharf, W., Perlinger, J., and Romanski, M. 2011. Concentrations of Environmental Contaminants in Herring Gull Eggs from Great Lakes Colonies in Michigan, 2002–2006. MI/DEQ/WRD—12/007. Michigan Department of Environmental Quality: Lansing, MI. 68 pp. https://www.michigan.gov/egle/-/media/Project/Websites/egle/Documents/Programs/WRD/SWAS/wildlife-2002-2006-gull.pdf?rev=545d8411e17045dcbe8419cf054a1cae&hash=4F8CE17C405392BA8D7371A3A61CEFC6
Burri, K., C. Gromke, and F. Graf. "Mycorrhizal fungi protect the soil from wind erosion: a wind tunnel study." Land Degradation & Development 24.4 (2013): 385-392.
Cain, A., Morgan, J. T., and Brooks, N. 2011. Mercury policy in the Great Lakes states: past successes and future opportunities. Ecotoxicology 20: 1500–1511. https://doi.org/10.1007/s10646-011-0764-4
Cheng, Shen, et al. "Elucidating the mechanisms underlying enhanced drought tolerance in plants mediated by arbuscular mycorrhizal fungi." Frontiers in Microbiology 12 (2021): 809473.
Chernyk, S., Hickey, J., and Benoche, I. 2002. PBDEs in Great Lakes Biota. Proceedings from Society of Environmental Toxicology and Chemistry: North America. Salt Lake City, UT: 16–20.
Clark, C.M., Simkin, S.M., Allen, E.B. et al. Potential vulnerability of 348 herbaceous species to atmospheric deposition of nitrogen and sulfur in the United States. Nat. Plants 5, 697–705 (2019). https://doi.org/10.1038/s41477-019-0442-8
Drevnick P. E., Roberts, A. P., Otter, R. R., Hammerschmidt, C. R. Klaper, R., and Oris, J. T. 2008. Mercury toxicity in livers of northern pike (Esox lucius) from Isle Royale, USA. Comparative Biochemistry Physiology Part C 147: 331–338. https://irma.nps.gov/DataStore/Reference/Profile/660638
Eagles-Smith, C.A., S.J. Nelson., C.M. Flanagan Pritz, J.J. Willacker Jr., and A. Klemmer. 2018. Total Mercury Concentrations in Dragonfly Larvae from U.S. National Parks (ver. 6.0, June 2021): U.S. Geological Survey data release. https://doi.org/10.5066/P9TK6NPT
Eagles-Smith, CA, JJ Willacker, CM Flanagan Pritz, AC Ellsworth. 2019. Total Mercury Concentrations in Fish from 31 National Parks, USA, 2015-2016. USGS Sensitive Data Release. https://irma.nps.gov/DataStore/Reference/Profile/2260288
Eagles-Smith, C.A., J.J. Willacker, S.J. Nelson, C.M. Flanagan Pritz, D.P. Krabbenhoft, C.Y. Chen, J.T. Ackerman, E.H. Campbell Grant, and D.S. Pilliod. 2020. Dragonflies as biosentinels of mercury availability in aquatic food webs of national parks throughout the United States. Environmental Science and Technology 54(14):8779-8790. https://doi.org/10.1021/acs.est.0c01255
Elliott SM, Brigham ME, Lee KE, Banda JA, Choy SJ, et al. 2017. Contaminants of emerging concern in tributaries to the Laurentian Great Lakes: I. Patterns of occurrence. PLOS ONE 12(9): e0182868. https://doi.org/10.1371/journal.pone.0182868
Elliott, Sarah M. and David D. VanderMeulen. 2017. A regional assessment of chemicals of concern in surface waters of four Midwestern United States national parks. Science of The Total Environment 579:1726-1735. https://doi.org/10.1016/j.scitotenv.2016.11.114
Evers, D. C., Kaplan, J. D., Meyer, M. W., Reaman, P. S., Braselton, W. E., Major, A., and Burgess, N., Scheuhammer, A. M. 1998. Geographic trend in mercury measured in common loon feathers and blood. Environmental Toxicology & Chemistry 17 (2): 173–183. https://irma.nps.gov/DataStore/Reference/Profile/52731
Evers, D. C., Wiener, J. G., Driscoll, C. T., Gay, D. A., Basu, N., Monson, B. A., Lambert, K. F., Morrison, H. A., Morgan, J. T., Williams, K. A., and Soehl, A. G. 2011a. Great Lakes Mercury Connections: The Extent and Effects of Mercury Pollution in the Great Lakes Region. Biodiversity Research Institute. Gorham, Maine. Report BRI 2011—18. 44 pp. Available at http://www.briloon.org/our-science-services/research-centers/center-for-mercury-studies-detail-page/mercury-center-opening-page/center-for-mercury-project-index/mercury-connections-landing-page/mercury-in-the-great-lakes-region.
Evers, D. C., Williams, K. A., Meyer, M. W., Scheuhammer, A. M., Schoch, N., Gilbert, A., Siegel, L., Taylor, R. J., Poppenga, R. and Perkins, C. R. 2011b. Spatial gradients of methylmercury for breeding common loons in the Laurentian Great Lakes region. Ecotoxicology 20: 1609–1625. https://doi.org/10.1007/s10646-011-0753-7
Geiser, Linda & Nelson, Peter & Jovan, Sarah & Root, Heather & Clark, Christopher. (2019). Assessing Ecological Risks from Atmospheric Deposition of Nitrogen and Sulfur to US Forests Using Epiphytic Macrolichens. Diversity. 11. 87. 10.3390/d11060087.
Geiser, Linda & Root, Heather & Smith, Robert & Jovan, Sarah & Clair, Larry & Dillman, Karen. (2021). Lichen-based critical loads for deposition of nitrogen and sulfur in US forests. Environmental Pollution. 291. 118187. 10.1016/j.envpol.2021.118187.
George, Eckhard, Horst Marschner, and Iver Jakobsen. "Role of arbuscular mycorrhizal fungi in uptake of phosphorus and nitrogen from soil." Critical reviews in biotechnology 15.3-4 (1995): 257-270.
Gorski, P. R., Cleckner, L. B., Hurley, J. P., Sierszen, M. E., and Armstrong, D. E. 2003. Factors affecting enhanced mercury bioaccumulation in inland lakes of Isle Royale National Park, USA. Science of the Total Environment 304 (1–3): 327–348. https://irma.nps.gov/DataStore/Reference/Profile/558137
Haro RJ and Others. 2013. Burrowing Dragonfly Larvae as Biosentinels of Methylmercury in Freshwater Food Webs. Environmental Science & Technology. 47(15):8148-8156. https://irma.nps.gov/DataStore/Reference/Profile/2230258
Hermanson, M. and Hites, R. 1990. Polychlorinated biphenyls in tree bark. Environmental Science & Technology 24: 666–671. https://irma.nps.gov/DataStore/Reference/Profile/94377
Horn KJ, Thomas RQ, Clark CM, Pardo LH, Fenn ME, Lawrence GB, et al. (2018) Growth and survival relationships of 71 tree species with nitrogen and sulfur deposition across the conterminous U.S.. PLoS ONE 13(10): e0205296. https://doi.org/10.1371/journal.pone.0205296
Kohut, R. 2004. Assessing the Risk of Foliar Injury from Ozone on Vegetation in Parks in the Great Lakes Network. Available at https://irma.nps.gov/DataStore/Reference/Profile/2181290.
Lee EH, Anderson CP, Beedlow PA, Tingey DT, Koike S, Dubois J, Kaylor SD, Novak K, Rice RB, Neufeld HS, Herrick JD. 2022. Ozone Exposure-Response Relationships Parametrized for Sixteen Tree Species with Varying Sensitivity in the United States. Atmospheric Environment. 284:1-16. https://irma.nps.gov/DataStore/Reference/Profile/2294221
Lilleskov, Erik A., et al. "Atmospheric nitrogen deposition impacts on the structure and function of forest mycorrhizal communities: a review." Environmental Pollution 246 (2019): 148-162.
McCoy K., M. D. Bell, and E. Felker-Quinn. 2021. Risk to epiphytic lichen communities in NPS units from atmospheric nitrogen and sulfur pollution: Changes in critical load exceedances from 2001‒2016. Natural Resource Report NPS/NRSS/ARD/NRR—2021/2299. National Park Service, Fort Collins, Colorado. https://doi.org/10.36967/nrr-2287254.
[NADP] National Atmospheric Deposition Program. 2018. NTN Data. Accessed January 20, 2022. Available at http://nadp.slh.wisc.edu/NADP/
[NPS] National Park Service, Inventory & Monitoring Program. 2010. Monitoring Persistent Contaminants at Isle Royale. Great Lakes Network Resources Brief. Available at: https://irma.nps.gov/DataStore/Reference/Profile/2196640
[NPS] National Park Service. 2022. Fish Consumption Advisories. https://www.nps.gov/subjects/fishing/fish-consumption-advisories.htm
Pierce RB, Harkey M, Lenzen A, Cronce LM, Otkin JA, Case JL, Henderson DS, Adelman Z, Nergui T, Hain CR. 2023. High-resolution air quality simulations of ozone exceedance events during the Lake Michigan Ozone Study. Atmospheric Chemistry and Physics, 23:9613–9635. https://doi.org/10.5194/acp-23-9613-2023
Porter, E., Blett, T., Potter, D.U., Huber, C. 2005. Protecting resources on federal lands: Implications of critical loads for atmospheric deposition of nitrogen and sulfur. BioScience 55(7): 603–612. https://doi.org/10.1641/0006-3568(2005)055[0603:PROFLI]2.0.CO;2
Risch M. R., Gay, D. A., Fowler, K. K., Keeler, G. J., Backus, S. M., Blanchard, P., Barres, J. A., Dvonch, J. T. 2012. Spatial patterns and temporal trends in mercury concentrations, precipitation depths, and mercury wet deposition in the North American Great Lakes region, 2002–2008. Environmental Pollution 161: 261–271. https://doi.org/10.1016/j.envpol.2011.05.030
Sandheinrich, M. B., Bhavsar, S. P., Bodaly, R. A., Drevnick, P. E., and Paul, E. A. 2011. Ecological risk of methylmercury to piscivorous fish of the Great Lakes region. Ecotoxicology 20: 1577–1587.
Saros, J. E. 2008. Determine critical nitrogen loads to boreal lake ecosystems using the response of phytoplankton. NPS Implementation Plan. 10 pp.
Scheuhammer, A. M. and Blancher, P. J. 1994. Potential risk to common loons (Gavia immer) from methylmercury exposure in acidified lakes. Hydrobiologia 279/280: 445–455.
Stroud CA, Ren S, Zhang J, Moran MD, Akingunola A, Makar PA, Munoz-Alpizar R, Leroyer S, Bélair S, Sills D, et al. 2020. Chemical Analysis of Surface-Level Ozone Exceedances during the 2015 Pan American Games. Atmosphere, 11(6):572. https://doi.org/10.3390/atmos11060572
Sullivan, T. J. 2016. Air quality related values (AQRVs) in national parks: Effects from ozone; visibility reducing particles; and atmospheric deposition of acids, nutrients and toxics. Natural Resource Report NPS/NRSS/ARD/NRR—2016/1196. National Park Service, Fort Collins, CO.
Swackhamer, D. L. and Hornbuckle, K. C. 2004. Assessment of Air Quality and Air Pollutant Impacts in Isle Royale National Park and Voyageurs National Park. NPS Report. Available at https://irma.nps.gov/DataStore/Reference/Profile/575135.
Thurman, E. M. and Cromwell, A. E. 2000. Atmospheric Transport, Deposition, and Fate of Triazine Herbicides and their Metabolites in pristine areas at Isle Royale National Park. Environmental Science and Technology 34 (15): 3079–3085.
Vucetich, L. M., Outridge, P. M., Peterson, R. O., Eide, R., and Isrenn, R. 2009. Mercury, lead and lead isotope ratios in the teeth of moose (Alces alces) from Isle Royale, U.S. Upper Midwest, from 1952 to 2002. Journal of Environmental Monitoring 11 (7): 1352–1359.
Vucetich, L. M., Vucetich, J. A., Cleckner, L. B., Gorski, P. R., and Peterson, R. O. 2001. Mercury concentrations in deer mouse (Peromyscus maniculatus) tissues from Isle Royale National Park. Environmental Pollution 114 (1): 113–118.
Last updated: September 30, 2024
