|
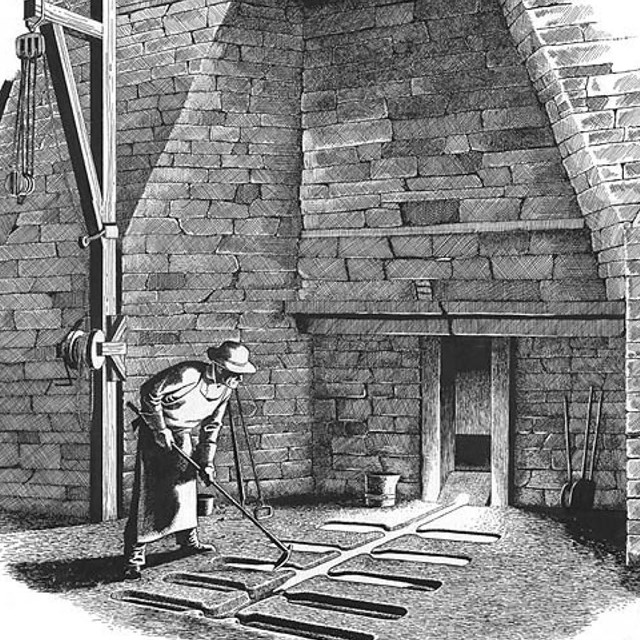
Iron making evolved over a few thousand years. Using the ancient "bloomery" method, iron ore was converted directly into wrought iron by heating the ore while at the same time melting the ore's impurities and squeezing them out with hand hammers. This is also called the "direct process." By the 1100s water-powered hammers replaced hand hammers for forging out bars of iron. Iron Making Process
|
Last updated: February 16, 2022