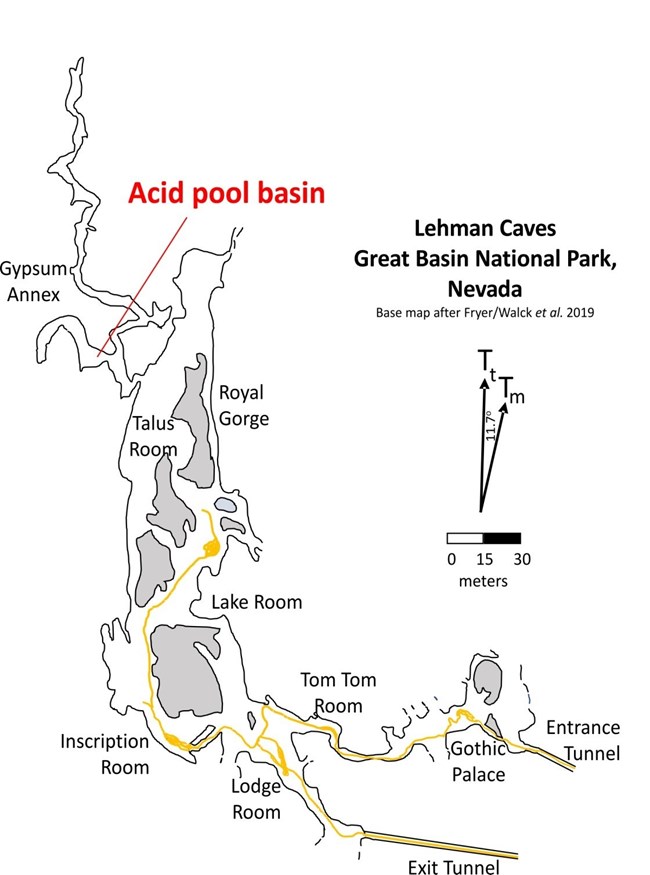
Lehman Caves is one of the key attractions in Great Basin National Park. In 1885 Absalom Lehman rediscovered the cave and made it into a show cave. Since at least 1960, the cave has been the subject of geologic investigation. Our knowledge of how caves are formed has come a long way since 1960, and we now know that there are multiple ways that caves can develop. The most common caves are epigenic, which means that they form near the surface of the earth as a result of limestone dissolution by carbonic acid. These caves are part of regional drainage systems. A great example is Mammoth Cave in Kentucky, and there are many thousands of similar caves in karst regions throughout the world. More than a dozen caves in Great Basin National Park are epigenic. In the 1970s, researchers in Carlsbad Caverns National Park realized that caves of the Guadalupe Mountains did not fit the epigenic model that had been established for Mammoth Cave. They discovered that most caves in the Guadalupe Mountains were formed by processes that took place inside the earth and were not connected to surface drainage systems. These caves are hypogenic, which means that they were formed by aggressive (acidic) water rising from deep-seated sources. They also found evidence of a significant chemical reaction mostly in the form of massive deposits of gypsum, a mineral that forms as a byproduct of the dissolution of limestone by sulfuric acid. Pioneering work by geologists Stephen Egemeier in the Kane Caves of Wyoming and Carol Hill in Carlsbad Cavern led to the sulfuric acid theory of cave formation and the subsequent recognition of similar caves all around the world. One place that contains evidence of development by sulfuric acid is Lehman Caves. Lehman Caves, at least in the Gypsum Annex (Fig. 1), displays evidence that it was formed when sulfuric acid dissolved marble in the Pole Canyon Limestone along natural fractures, enlarging them and creating cave passages. Gypsum, the byproduct of the reaction between sulfuric acid and limestone (or marble), has mostly been removed from the known parts of Lehman Cave. However, there are telltale signs that gypsum was once present. These signs include an array of bedrock features (speleogens) that formed when the sulfuric acid event was happening several million years ago. These speleogens include rillenkarren, pseudo-scallops, incised water lines or notches, overhung pool margins (Fig. 2). Taken together, these features are indicators that there was once a pool of water that contained dilute sulfuric acid. We call these ancient pool sites acid pool basins. 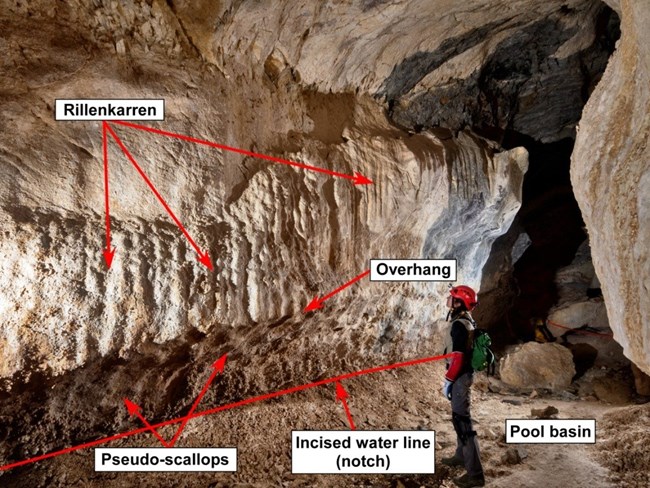
Photo by Dave Bunnell. In active sulfuric acid caves such as Cueva de Villa Luz in Mexico, hydrogen sulfide gas is released from upwelling water in warm springs at the water table. The water table is a horizontal plane below which all of the voids in the bedrock are filled with water. The rocks above the water table may contain some water but voids are mostly filled with air. Upwelling spring water carrying dissolved hydrogen sulfide generally does not contain dissolved oxygen. This is because hydrogen sulfide in the spring water combines with available oxygen to form sulfuric acid which immediately reacts with carbonate (limestone or marble) and is neutralized. This process scavenges all free oxygen (O2) from the spring water, and the remaining hydrogen sulfide and water molecules comprise weak sulfurous acid. 2H+ + S- + 2O2 çè H2SO4 Hydrogen Oxygen Sulfuric Sulfide acid When warm, upwelling water containing dissolved hydrogen sulfide reaches the water table, some water vapor and some hydrogen sulfide are released into the cave atmosphere. Because of heat derived from the warm or hot springs and also from the chemical reaction, the cave atmosphere is warmer than the surrounding bedrock. Water vapor that comes in contact with cool passage walls condenses, forming a coating of moisture that contains dissolved oxygen acquired from the cave atmosphere. Hydrogen sulfide is highly soluble in water and does not like to exist as a gas. Consequently, the hydrogen sulfide released from the agitated spring water is quickly re-absorbed into the oxygen-rich condensed water on passage walls, forming sulfuric acid. If the acid rests on a limestone wall, there is an immediate reaction between the acid and the limestone and moist acidic gypsum paste is formed. This reaction can be enhanced by certain acid-tolerant microbes. CaCO3 + H+ SO42- + 2H2O çè CaSO4 . 2H2O + HCO3- Calcite Sulfuric Water Gypsum Bicarbonate Acid Ion The condensation water on the cave wall is similar to the droplets that form on the outside of a drinking glass filled with a cold liquid on a warm, humid day. When a lot of moisture condenses on the glass, gravity overcomes surface tension and drops of water flow down the side, leaving a small puddle on the tabletop. This process also happens inside actively forming sulfuric acid caves where the side of the drinking glass is analogous to the cave wall and the puddle on the tabletop is equivalent to the water that accumulates in the pool basin. Where there is a large surface area in the cave passage above a pool, more condensation and, therefore, more sulfuric acid accumulates. This acidic condensate water flows down the wall in channels, much like the water dripping down the side of the cold drinking glass. The acidic water flowing down the cave wall dissolves grooves known as rillenkarren (Fig. 2). At sites where moisture is less likely to accumulate in sufficient quantity to flow down the wall (e.g., undercuts in the wall), acidic gypsum paste accumulates until it falls under the influence of gravity. The place where the gypsum originated is a concave, cuspate depression that superficially resembles a cave scallop formed by flowing water (Fig. 2). Since the term cave scallops is usually associated with flowing water, we have named the cuspate depressions formed by sulfuric acid dissolution pseudo-scallops. If the passage floor is a closed depression, condensed water accumulates and forms a pool. Any sulfuric acid in the pool water dissolves limestone from the bedrock floor and walls and gradually enlarges the basin. Acidic water in gypsum paste that falls into the pool continuously supplies more acid, which is rapidly neutralized. Most of the reaction between bedrock and acid occurs at or near the upper surface of the water, near the edges of the pool where dissolution of the wall causes a constant widening of the basin. This creates an overhung wall, or solution notch around the margin of the pool basin (Fig. 2).) One of the basic premises of the hypogene sulfuric acid theory of cave formation is that most of the dissolution of limestone occurred at or slightly above the water table. Today, Lehman cave lies a significant distance above the water table, so hydrologic conditions must have been different at the time the acid pool basin was active. The water table must have been higher. The floor of the adjacent Snake Valley, if it existed at that time, would have been higher than it is today. The Snake Range is probably higher today than it was when the cave was formed due to regional, basin-and-range uplift and/or isostatic rebound caused by removal of a large mass of rock from the top of the uplift. Regional detachment faulting exposed the core of the Snake Range uplift and continued erosion of strata above the cave host rocks would have resulted in uplift and a lowering water table. Thus, the lowering water table was due to a combination of tectonic uplift, isostatic rebound, and erosion. A second premise of cave development by sulfuric acid is that there had to be a source of hydrogen sulfide in order for the acid to be formed. At present, springs with hydrogen sulfide are not present in or near the southern Snake Range, but they are present elsewhere in the Great Basin of Nevada and Utah. Clearly, there are many significant questions that need to be answered before we understand how Lehman Caves was formed. One thing that is clear is that studying Lehman Caves helps us recognize that these questions exist. Continued research will help us understand how Lehman and other caves in Great Basin National Park were formed and may lead to a better understanding of the geologic history of the Great Basin over the last 15 million years. References: DuChene, H.R. and Hose, L.D., 2020, Trip report for 2-4 March 2020 Lehman Cave, Great Basin National Park, Baker, NV: Unpublished report to Great Basin National Park, 15 p. Egemeier, S.J.,1971, A comparison of two types of solution caves: Unpublished report to Carlsbad Caverns National Park, April 12: 7 p. Egemeier, S.J., 1973. Cavern development by thermal waters with a possible bearing on ore deposition: Unpublished PhD dissertation, Stanford University: 88 p. Egemeier, S.J., 1987. A theory for the origin of Carlsbad Cavern: NSS Bulletin v.49, n.2, p. 73-76. Jagnow, D.H., Hill, C.A., Davis, D.G., DuChene, H.R., Cunningham, K.I., Northup, DE, Queen, J.M., 2000, History of sulfuric acid theory of speleogenesis in the Guadalupe Mountains, New Mexico: Journal of Cave and Karst Studies, v. 62, n.2, p. 54-59.
Return to Cave/Karst page
|
Last updated: September 1, 2021
