
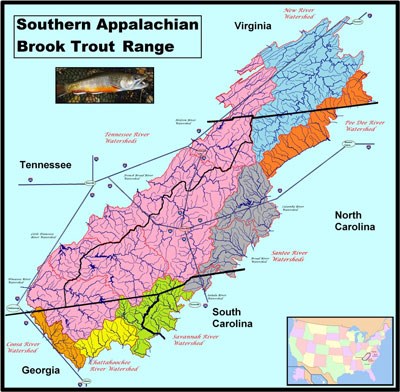
NPS photo The range of the Southern Appalachian Brook Trout (SABT) is greater than just the Great Smoky Mountains National Park (GSMNP) (see Figure 1), but GSMNP has been a pioneer in research related to brook trout genetics, population monitoring, restoration, and other brook trout studies by local, state, and federal agencies and a myriad of universities.
The SABT has experienced severe declines within its range for a number of reasons. One of the most significant, at least in the GSMNP, has been the episodic acidification of surface waters. Many studies have been conducted over the last decade looking at the issue and its impact on the physiology and survivability of the SABT. Environmental stressors such as headwater water chemistry changes, predominantly pH changes, have been shown to be the major source of headwater declines in brook trout range in GSMNP (Wesner et al. 2011). What this excerpt will focus on is the physiological mechanisms used by SABT to cope with acidosis in their environment.
Southern Appalachian Brook Trout Foundation photo Increased industrial emissions were caused by the burning of fossil fuels that release SO2, NO, NO2, and NH3 into the atmosphere, where it can attach water vapor and fall with precipitation in the form of H2SO4, HNO3, and NH4+ (Driscoll et al. 2003). GSMNP receives elevated rates of atmospheric acid deposition in comparison with other areas on the east coast, resulting in increased episodic stream acidification events, adding to the acidification of soil and surface waters in the park. Episodic stream acidification occurs when increased rates of atmospheric acid deposition occur, bringing increased precipitation to soils and water bodies, resulting in periods of increased stream flow and decreased water pH. Areas with heavy year-round rates of atmospheric acid deposition tend to have decreased soil pH. By decreasing the number of base cations (or positive ions like calcium, potassium and sodium) in the local geology, this limits buffering capabilities, and exacerbates the pH problem. Organic acids also contribute to the acidification of streams in the form of natural plant nitrates carried from surface soils into stream channels.
Acids leach into poorly buffered streams of the GSMNP through wet deposition (acid rain), dry deposition (pollutants that stick to leaves during dry periods, but are flushed from these and other surfaces during rain events), and naturally occurring organic acids in soils. When these acids run off into the watershed, they cause rapid declines in stream pH (Driscoll et al. 2003, Neff et al. 2009). Acidification events create a rapid change in stream pH and a general imbalance in the environmental ions available for ion exchange. The mechanism of acid stress in fishes is generally recognized as an ion regulatory disturbance that can lead to circulatory collapse and in extreme cases death.
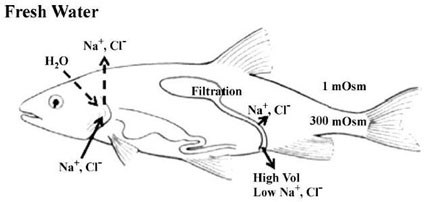
Environmental acidifications are very taxing events on normal fish physiology. Fish are highly sensitive to even minute changes in environmental pH, which can result in changes in enzyme function. (Claiborne et al. 2002) Acid/base regulation is a greater challenge for fish than it is for terrestrial organisms primarily because of the use of water as a respiratory medium in contrast to air. As a result fish must ventilate their gills at a high rate in order to meet normal oxygen needs. The smallest of changes in environmental pH may result in relatively large changes in blood pH for a fish. To counteract any abrupt changes in internal pH, fish rely on the direct transfer of acid/base relevant ions (H+, HCO3-, NH4+, OH-) across primarily gill epithelial surfaces, but also the kidney and intestine have been shown to be involved (Evans, et al. 2005).
A variety of mechanisms have been postulated to be involved in acid/base relevant ion exchange. In freshwater fish, the epithelial mechanism associated with the excretion of excess acid has been shown to be the proton ATPase (H+-ATPase). (Perry et al. 2003, Evans et al. 2005)
Brian Patrick Mikeworth photo The current range of the SABT has been created by a variety of historic and current geologic, geographic, and anthropogenic influences as well as recent environmental factors on the species, such as habitat loss and degradation, competition with nonnative fishes, climate change, and atmospheric acid deposition (Galbreath et al. 2001, Aunins 2015). Some declines of SABT populations can be originally attributed to deforestation of their native habitats by timber companies, the lack of forest decreased stream quality due to sediments and runoff flowing into the streams with the absence of a riparian buffer to control it (Karas 1997). The lack of shade that the recently logged timbers creates also allowed water temperatures to rise, forcing the fish to move to more shaded, cooler zones or die (Aunins 2015). The logging also impacted the riparian buffer which then impacts the chemistry of the streams.
The water quality of some streams and rivers, which SABT inhabit inside of the park, regularly fall outside of the standards set by the "Clean Water Act" putting these fish at risk of acidosis (GSMNP Water Quality Annual Report 2011). In fact in 2008, 12 Tennessee streams were listed as "Impaired" on the state 303d list due to low stream pH (mean pH values less than 6.0). In many areas of the park with SABT populations, there are high elevation, small basin watersheds, which typically have lower stream sodium content, low acid neutralizing capacity (ANC) of the stream and soil, and high water flow in storm events (Neff et al. 2013). Within these high elevation watersheds of GSMNP, it is believed that episodic acidification of streams associated with atmospheric acid deposition is the greatest threat contributing to the loss of SABT.
This difference in water chemistry around the park can create difficulties when completing water quality assessments and their impacts on the SABT populations, especially since there are adaptive differences within GSMNP SABT populations let alone across their entire range based on environmental adaptations and habitat differences. In other studies unique stream specific adaptations have been noted in SABT populations from one stream to another within the park. Though no study has been found to support the hypothesis it would be logical to assume there would also be differences across SABT range as well. Given this data and assumption it could be concluded that separate SABT populations would react differently to short and long term pH change events, therefore making a definitive comparison and conclusion difficult without more study.

Minshu Deng photo The SABT is constantly undergoing changes in its internal pH due to atmospheric acid deposition affecting the acid balance of its native streams. These constant changes make it very difficult for these animals to maintain a systemic pH homeostasis or balance, although it is necessary for their survival. The aim of this research is to provide physiological information from SABT that can assist GSMNP in determining toxicity thresholds of the various acid deposition compounds. These data can be used to set air quality standards to guide emission reductions that will restore and maintain viable, healthy SABT populations within the park. To provide these data, the GSMNP fisheries department focuses a portion of their fish and water quality monitoring efforts on streams known to be low in sodium content in order to assess fish and stream health. These data will guide regulators and park managers in developing strategic GSMNP plans that will reduce acidification of park streams and provide the habitat conditions necessary to protect and preserve SABT populations within and across its borders for future generations.

NPS Photo Suggested Links
Prepared by Guy Jester.
|
Last updated: August 17, 2015
