Cave OriginOur understanding of the origin of Lehman Caves has changed a great deal over the last few years due to additional research. Here is an excerpt from Louise Hose's 2018 "The Geologic Story of Lehman Caves."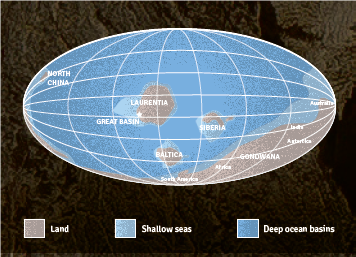
Image created by The Design Minds. 1. Formation of the Pole Canyon Limestone, which contains Lehman Caves. This rock layer began as limestone that originated from a warm, shallow sea about 550 million years ago near the equator. This limestone layer was covered and uncovered periodically. Later, the rock layers drifted north to their present location. When the Basin and Range province was made, the rock was put under a lot of heat and pressure, making it into a low grade marble. The mountain-building phase created many fractures in the rock. 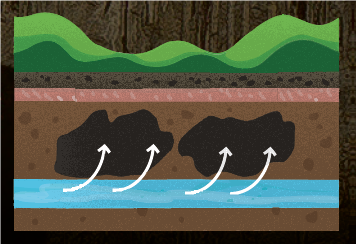
Image created by The Design Minds 
NPS photo
4. Condensation corrosion. This last phase has only recently been identified. As you go through Lehman Caves, you may notice that many of the speleothems look like they’ve been eaten away. This is due to air, seasonally high in carbon dioxide, dissolving away speleothems and marble. This process may only have started within the last ten thousand years, as the climate got drier.
Return to the Caves/Karst page.
NPS IMAGE |
Last updated: August 28, 2021
