Part of a series of articles titled Yellowstone Science - Volume 27 Issue 1: Vital Signs - Monitoring Yellowstone's Ecosystem Health.
Article
SHORT: An Uncertain Future: the Persistence of Whitebark Pine in the Greater Yellowstone Ecosystem

(July, 2018; NPS PHOTO - E. SHANAHAN).
An Uncertain Future: the Persistence of Whitebark Pine in the Greater Yellowstone Ecosystem
by Erin K. Shanahan
If ever I was to love a tree, this is the tree (figure 1) that would own my heart. Enduring gracefully at the base of a narrow, high-elevation cirque in the Wind River Range, it is a challenging off-trail scramble to be in its presence. My first encounter with this massive whitebark pine was in July 2014. Located just a stone’s throw from our monitoring plot, I felt compelled to pay homage to this incredible specimen that has clearly withstood hardship. While I do not know its age, I would posit this towering whitebark pine has been rooted in this location for more than 500 years. The extensive fire scar at its base, combined with its age, confirm that this timeworn tree has survived periods of immense environmental stress.
Throughout the past decade, I have personally witnessed the death of thousands of whitebark pine trees due to the voracious appetite of a diminutive, native bark beetle, the mountain pine beetle. How this tree and its neighboring whitebark pine have escaped beetle attack astounded me. In addition, the pathogen white pine blister rust had yet to infiltrate this oasis. While this individual has stood tall and chronicled the story of environmental change within its tree rings, others of its species have been less fortunate.
Whitebark pine is considered a keystone and foundation species that exerts strong influences on the biodiversity and productivity of high-elevation and subalpine communities in the Pacific Northwest and northern Rocky Mountains. Because of these traits and the multiple concurrent threats it faces, the selection of whitebark pine as a regional, cross-jurisdictional (e.g., Bureau of Land Management, National Park Service, and U.S. Forest Service) vital sign has been widely embraced.
Whitebark Pine Declines
Substantial declines in whitebark pine have been documented throughout its range (Logan et al. 2009). Decreases can be attributed to a number of factors acting individually or in concert: mountain pine beetle, white pine blister rust (caused by the introduced fungus Cronartium ribicola), more frequent and intense wildfires (“Nowcasting and Forecasting Fire Severity in Yellowstone,” this issue), and climate-induced drought. These agents all pose significant risk to the persistence of whitebark pine populations on the landscape; as a result, whitebark pine was listed as a candidate species under the Endangered Species Act in 2011.
Mountain pine beetles are one of most aggressive and damaging bark beetles of western pine forests (RMR, FHP 2010). The behavior of mountain pine beetle is strongly tied to temperature (Jewett 2010). For the most part, temperature constraints have, until recently, limited mountain beetle to lower elevations (Logan and Bentz 1999, Bentz et al. 2010). Cold temperatures have also restricted mountain pine beetle to a life cycle that requires multiple years to complete a generation (Logan and Powell 2004, Bentz et al. 2015). Historically, cold, high-elevation temperatures prevented synchronized outbreaks of mountain pine beetle, thus keeping the beetles and whitebark pine trees separated (Raffa et al. 2013). But recent warming at whitebark pine elevations has enabled beetles to move upslope and attack whitebark pine, a species that, from an evolutionary perspective, did not have to evolve strong defenses to combat beetles (Raffa et al 2013). From 2006 to 2008, above-average temperatures exceeded a cumulative temperature threshold, enabling mountain pine beetle to shift from a multi-year to a single-year life cycle, expediting reproduction (Carroll et al. 2006). This collapse from a multi-year to single-year life cycle resulted in large, synchronized attacks on whitebark pine.
The Perfect Storm
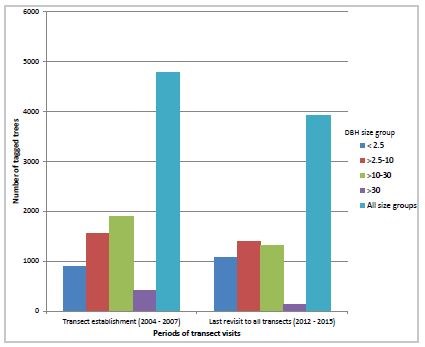
The 2006-2008 warming period combined with an abundant food supply was the “perfect storm” for the eruption of mountain pine beetles at epidemic levels across the Greater Yellowstone Ecosystem (GYE). The Greater Yellowstone Inventory and Monitoring Network’s monitoring program documented a steady increase in mountain pine beetle-associated mortality with a substantial increase in recorded deaths of monitored trees from 2008 to 2009. In 2009, an early season cold snap likely killed mountain pine beetle larvae before they had become cold hardened (Dooley and Six 2015, Shanahan et al. 2016). The consequences of this weather event and a significantly depleted food supply (beetles had literally eaten themselves out of house and home) in many areas of the GYE likely returned mountain pine beetle populations to pre-warming or endemic levels. Unfortunately, the mortality attributed to the three-year mountain pine beetle outbreak resulted in an overall reduction in the number of larger, reproducing trees and a shift to smaller-sized, typically non-reproducing trees in the remaining whitebark pine stands (figure 2; Shanahan et al. 2016).
Unlike the rapid mortality due to mountain pine beetle, white pine blister rust (blister rust) infection is more gradual but potentially as lethal. Blister rust infection is ubiquitous throughout the GYE, with infection levels varying across the region (Shanahan et al. 2017). Temperature and moisture are key factors in blister rust dispersal and successful infection (Kendall and Keane 2001). Infections typically initiate when airborne spores enter open stomata in needles near the crown or top of a tree. Over time, an infection can expand or transition from the needles to an adjacent branch and eventually to the trunk or bole. Bole infections are generally more debilitating or lethal to the tree (Campbell and Antos 2000, McDonald and Hoff 2001). Once infected, smaller whitebark pine have a greater likelihood of mortality than larger trees (Shanahan et al. 2016). Smaller trees may be more susceptible because they have fewer and shorter branches, which reduce the distance an infection has to travel from branch to bole. In comparison, larger trees may resist infection expansion by shedding branches or “walling off” infections (Tomback et al. 1995).
Uncertain Future
There are contrasting hypotheses on the vulnerability of understory whitebark pine to blister rust exposure and the mechanisms affecting infection transmission. Some have hypothesized that infection is less likely in smaller trees, which are smaller targets and benefit from an umbrella-like protection by the overstory (Campbell and Antos 2000, Smith and Hoffman 2000, Kearns and Jacobi 2007). Others suggest the microclimate within the understory of an intact stand plays a critical factor in increasing susceptibility to infection transfer by trapping and increasing humidity (Tomback et al. 1995, Smith et al. 2008, Mahalovich 2013). The recent mortality of large whitebark pine trees has fragmented the overstory canopy in stands across the GYE. As a result, if the first hypothesis is correct, an overall increase in blister rust infection of understory whitebark pine where the sheltering qualities of an intact, multi-structural forest have been disrupted may be seen. Alternatively, if the second hypothesis is correct, the loss of upper canopy trees may have the effect of altering the understory environment such that the critical conditions necessary for the complex transfer of blister rust spores to susceptible understory hosts is now less favorable (open canopy and decreased humidity). This presents an interesting situation where the impacts of the recent mountain pine beetle outbreak may facilitate or, conversely, impede the future dispersal patterns of blister rust in the remaining whitebark pine population.
Though multiple factors contribute to a tree’s susceptibility to infection and the likelihood that an infection moves from one part of a tree to another, environmental conditions play a substantial role. For example, weather conditions affect many aspects of blister rust transmission, and within the GYE these conditions can vary temporally and spatially (Mahalovich 2013). Seasonal fluctuations in weather patterns not only enhance (i.e., warmer temperatures at higher elevations create a longer growing season) or hamper (i.e., colder temperatures shorten the growing season) blister rust spore development and dispersal but can also promote or inhibit infection transition on an infected tree (Kearns et al. 2009). From 2004 to 2015, I documented larger trees transitioning from a canopy-infection to a bole-infection at a high rate (48% of monitored trees in our study; Shanahan 2015). Furthermore, among smaller sized canopy-infected trees, there was a 50% chance that a canopy infection expanded to the bole in just a four-year time span (Shanahan 2015). While infected whitebark pine can persist for decades, bole infections negatively impact overall vigor and reproductive potential by precluding the flow of vital nutrients necessary to sustain normal tree functions, healthy foliage, and cone production (Maloney et al. 2012).
In recent years, whitebark pine populations across its range have been compromised by the impacts of mountain pine beetle, blister rust, wildfire, and climate-induced drought. For some regional and local populations, conditions at or nearing an ecological tipping point may exist. In an ecological context, a tipping point is an irreversible change from one ecosystem state to another (e.g., desertification and marine fisheries collapse) such that conditions cannot be reversed. As temperatures continue to climb across all elevations of the GYE, the temperature-induced changes to the mountain pine beetle reproductive cycle coincident with the massive mortality of large trees witnessed from 2006 to 2008 may be the new normal. All remaining trees are susceptible to blister rust, and warming conditions are also likely to bring hotter and more frequent fires (Westerling et al. 2006). If conservation management actions designed to curb one or more of these factors are to be implemented in the GYE (GYCCWPS 2011), it is essential that scientists work collaboratively to determine the window of management opportunity so intervention can be most effective. Although some of the benefits of our region-wide monitoring program are already helping to understand the complexities of recent threats to whitebark pine, continued monitoring will serve as an enduring resource for decision makers today and into the future.
In July 2018, I revisited the enclave where my favorite whitebark pine resides, returning with mixed emotions and some hesitation. On the hike out in 2014, I came across an active mountain pine beetle outbreak a mere 300 feet (91 m) downslope from this untainted stand. Given my knowledge of the dispersal capabilities of mountain pine beetle, my fear was that even this astounding monarch may succumb to a fatal attack. Well, my anxiety was for naught (figure 1); I will keep my fingers crossed until my next visit in 2022.
Literature Cited
Bentz, B.J., J. R´egni`ere, C.J. Fettig, E.M. Hansen, J.L. Hayes, J.A. Hicke, R.G. Kelsey, J.F. Negr´on, and S.J. Seybold. 2010. Climate change and bark beetles of the western United States and Canada: direct and indirect effects. Bioscience 60:602– 613.
Bentz, B.J., C. Boone, and K.F. Raffa. 2015. Tree response and mountain pine beetle attack preference, reproduction and emergence timing in mixed whitebark pine and lodgepole pine stands. Agricultural and Forest Entomology 17:421–432.
Campbell, E.M., and J.A. Antos. 2000. Distribution and severity of white pine blister rust and mountain pine beetle on whitebark pine in British Columbia. Canadian Journal of Forest Research 30:1051-1059.
Carroll, A.L., J. Régnière, J.A. Logan, S.W. Taylor, and J.A. Powell. 2006. Impacts of climate change on range expansion by the mountain pine beetle. Mountain Pine Beetle Initiative working paper 2006-14. Pacific Forestry Center, Natural Resources Canada, Canadian Forest Service, Victoria, British Columbia, Canada.
Dooley, E.M., and S.L. Six. 2015. Severe white pine blister rust infection in whitebark pine alters mountain pine beetle (Coleoptera: Curculionidae) attack density, emergence rate, and body size. Environmental Entomology 44:1384–1394.
Greater Yellowstone Whitebark Pine Monitoring Working Group (GYWPMWG). 2011. Interagency whitebark pine monitoring protocol for the Greater Yellowstone Ecosystem, Version 1.1. Greater Yellowstone Coordinating Committee, Bozeman, Montana, USA.
Greater Yellowstone Coordinating Committee Whitebark Pine Subcommittee (GYCCWPS). 2011. Whitebark pine strategy for the Greater Yellowstone Area. Greater Yellowstone Coordinating Committee, Bozeman, Montana, USA.
Jewett, J.T., R.L. Lawrence, L.A. Marshall, P.E. Gessler, S.L. Powell, and S.L. Savage. 2010. Spatiotemporal relationship between climate and whitebark pine mortality in the Greater Yellowstone Ecosystem. Forest Science 57:320-335.
Kearns, H.S., and W.R. Jacobi. 2007. The distribution and incidence of white pine blister rust in central and southeastern Wyoming and northern Colorado. Canadian Journal of Forestry Research 37:462-472.
Kearns, H.S., W.R. Jacobi, and B.W. Geils. 2009. A method for estimating white pine blister rust canker age on limber pine. Forest Pathology 29:177-191.
Kendall, K.C., and R.E. Keane. 2001. Whitebark pine decline: infection, mortality, and population trends. Pages 221-242 in D.F. Tomback, S.F. Arno, and R.E. Keane, editors. Whitebark pine communities: ecology and restoration. Island Press, Washington, D.C., USA.
Logan, J., and B. Bentz. 1999. Model analysis of mountain pine beetle (Coleoptera: Scolytidae) seasonality. Environmental Entomology 28:925–934.
Logan, J.A., and J.A. Powell. 2004. Modelling mountain pine beetle phenological response to temperature. Pages 210–222 in T.L. Shore, J.E. Brooks, and J.E. Stone, editors. Mountain pine beetle symposium: challenges and solutions. Pacific Forestry Center, Natural Resource Council, Canadian Forest Service, Victoria, British Columbia, Canada.
Logan, J.A., W.W. Macfarlane, and L. Willcox. 2009. Effective monitoring as a basis for adaptive management: a case history of mountain pine beetle in Greater Yellowstone Ecosystem whitebark pine. iForest-Biogeosciences and Forestry 2:19-22.
Maloney, P.E., D.R. Volger, C.E. Jensen, and A.D. Mix. 2012. Ecology of whitebark pine populations in relation to white pine blister rust infection in subalpine forests of Lake Tahoe Basin, USA: implications for restoration. Forest Ecology and Management 280:166-175.
Mahalovich, M.F. 2013. Grizzly bears and whitebark pine in the Greater Yellowstone Ecosystem. Future status of whitebark pine: blister rust resistance, mountain pine beetle, and climate change. Report 2470 RRM-NR-WP-13-01. U.S. Department of Agriculture, Forest Service, Northern Region, Missoula, Montana, USA.
McDonald, G.I., and R.J. Hoff. 2001. Blister rust: an introduced plague. Pages 193–220 in D.F. Tomback, S.F. Arno, and R.E. Keane, editors. Whitebark pine communities ecology and restoration. Island Press, Washington, D.C., USA.
Raffa, K.F., E.N. Powell, and P.A. Townsend. 2013. Temperature-driven range expansion of an irruptive insect heightened by weakly coevolved plant defenses. Proceedings of the National Academy of Sciences USA 110:2193–2198.
Rocky Mountain Region, Forest Health Protection. 2010. Field guide to diseases and insects of the Rocky Mountain Region. Gen Tech. Rep. RMRS-GTR-241. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fort Collins, Colorado, USA.
Shanahan, E.K. 2015. Trends in whitebark health in the Greater Yellowstone Ecosystem. Thesis. Montana State University, Bozeman, Montana, USA.
Shanahan, E., K.M. Irvine, D. Thoma, S. Wilmoth, A. Ray, K. Legg, and H. Shovic. 2016. Whitebark pine mortality related to white pine blister rust, mountain pine beetle outbreak, and water availability. Ecosphere 7(12):e01610.
Shanahan, E., K. Legg, and R. Daley. 2017. Status of whitebark pine in the Greater Yellowstone Ecosystem: a step-trend analysis with comparisons from 2004 to 2015. Natural Resource Report NPS/GRYN/NRR—2017/1445. National Park Service, Fort Collins, Colorado, USA.
Smith, J.P., and J.T. Hoffman. 2000. Status of white pine blister rust in the Intermountain West. Western North American Naturalist 60:165-179.
Smith, C.M., B. Wilson, S. Rasheed, R. Walker, T. Carolin, and B. Sheppard. 2008. Whitebark pine and white pine blister rust in the Rocky Mountains of Canada and northern Montana. Canadian Journal of Forestry Research 38:982-995.
Tomback, D.F., J.K. Clary, J. Koehler, R.J. Hoff, and S.F. Arnos. 1995. The effects of blister rust on postfire regeneration of whitebark pine: the Sundance burn of northern Idaho (U.S.A.). Conservation Biology 9:654–664.
Westerling, A.L., H.G. Hidalgo, D.R. Cayan, and T.W. Swetnam. 2006. Warming and earlier spring increase western US forest wildfire activity. Science 313:940–943.
Last updated: August 20, 2024
