Ticks were rarely found around shelters or tenting areas, and were 14.5 times more likely to be encountered on the trail itself.
Get PDF
Download PDF of this article
Abstract and key words
Abstract: The Appalachian National Scenic Trail (AT) runs 3,520 km (2,187 mi) from northern Georgia to northern Maine, traversing 14 states where Lyme disease and other tickborne diseases are endemic or emerging. Approximately 2–3 million visitors hike the AT annually, including through-hikers who spend five to six months on the trail in spring through early fall, when common tick species are active. Disease vector tick surveillance was conducted from April through August 2013 at 42 randomly selected AT shelter areas along a south-to-north transect covering the full length of the AT. Tick abundance at shelters and tenting areas was compared with tick abundance on the AT itself, and the collected ticks were tested for common bacterial pathogens. Human-biting tick species collected comprised Ixodes scapularis, Amblyomma americanum, Amblyomma maculatum, and Dermacentor variabilis. Human pathogens Borrelia burgdorferi and Rickettsia montanensis were detected in tested ticks. Tick abundance on the trail was low overall (2.8 ticks per 1,000 m2 sampled), but exceeded tick abundance in shelters and tenting areas by 14.5 times. No ticks were collected south of Virginia or north of Massachusetts, or above 829 m (2,720 ft) in elevation, which suggests that season and elevation are significant determinants of the risk of hiker exposure to questing ticks on the AT. Such information should be included in future health messaging to hikers along with preventive measures. Management issues are discussed.
Key words: Appalachian Trail, Ixodes scapularis, Lyme disease, prevention
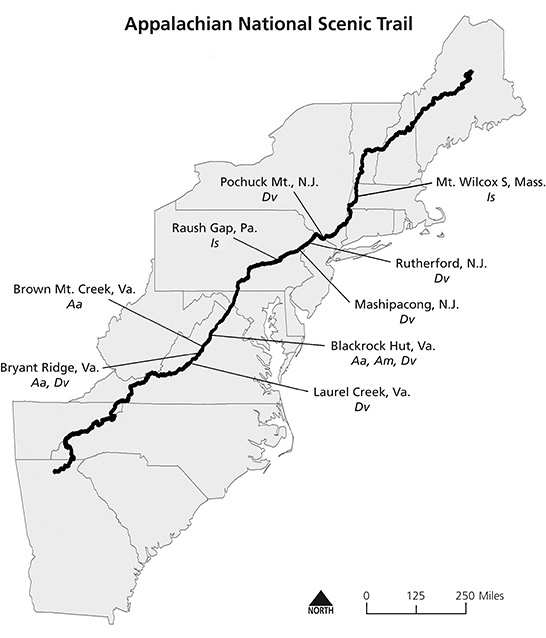
THE APPALACHIAN NATIONAL SCENIC TRAIL (AT) is a moderate-elevation, 3,520 kilometer-mile-long (2,187 mi) hiking trail in the Appalachian Mountains extending from Georgia to Maine in the United States (fig. 1). Each year, 2–3 million people hike portions of the trail. In 2013, approximately 2,700 northbound through-hikers started out on the trail in Georgia in late winter or spring, taking five to six months to complete the hike (http://www.appalachiantrail.org/about-the-trail). During the hiking season, hikers and trail maintainers are at risk of exposure to ticks and tickborne disease agents, especially the Lyme disease bacterium Borrelia burgdorferi.

Graham Hickling
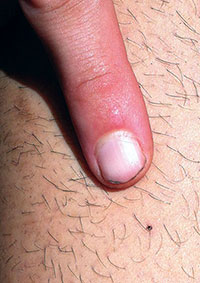
About 33,000 cases of Lyme disease were reported in the United States in 2011, many in the New England and mid-Atlantic eastern states traversed by the AT (CDC 2013). Reported cases underestimate the true incidence, which may exceed 300,000 annually (Mead 2013). The National Park Service does not know how many cases of Lyme disease are initiated on the trail; however, 9% of respondents to an AT hiker survey reported they had been diagnosed with Lyme disease (Knoll et al. 2014). In the eastern United States, B. burgdorferi is transmitted to humans via the black-legged or “deer” tick, Ixodes scapularis (fig. 2). Juvenile (nymphal) ticks cause the most cases of Lyme disease and are about the size of a poppy seed (fig. 3). In the Northeast, most tick bites involve I. scapularis or the American dog tick, Dermacentor variabilis. In the Southeast, most bites are from the lone star tick, Amblyomma americanum (Stromdahl and Hickling 2012). These latter two tick species transmit ehrlichial and rickettsial diseases, but not Lyme disease.
Karl Ford
Tick activity is highly seasonal: nymphal I. scapularis and adult A. americanum are active in spring and summer, whereas adult I. scapularis are active from fall through spring (Diuk-Wasser et al. 2006). In addition, ticks tend to avoid higher elevations; in a study of mid-Atlantic states, no I. scapularis were found above 530 m (1,739 ft) (Bunnell et al. 2003). The Centers for Disease Control and Prevention published Lyme disease case maps for the United States in 2013 that are influenced by population centers. Consequently, those maps are of limited utility in determining the likelihood of AT hikers being exposed to ticks along different sections of the trail, particularly in rural areas where population density is relatively low. Furthermore, hikers may sleep in, or camp near, primitive camping shelters on the AT. These shelters are used by rodents that are hosts for ticks and reservoirs for tickborne human pathogens.
Lyme disease risk among AT hikers
Lyme disease causes a flulike illness and, in 10–20% of treated cases and 60% of untreated cases, can result in debilitating, chronic Lyme disease syndrome. Symptoms include significant joint swelling and arthritis. A smaller percentage of cases experience neurological problems. As the manager of the Appalachian National Scenic Trail, the National Park Service supported this research to help determine Lyme disease risk to AT hikers.
Several management issues related to this public health concern drove our research on the Appalachian Trail. The first concerned the scope of the Lyme disease problem among AT users. There are no data on tick abundance around shelters and only limited data on their abundance on the trail itself (Oliver and Howard 1998). A second issue was whether or not the camping shelters might provide harborage for the reservoir of Lyme disease (mice and rodents). No data of this type were known. A final concern was to better identify tickborne disease-prevention measures, including trail vegetation trimming and hiker education. The objective of this research, therefore, was to assess species distribution and abundance of ticks at representative shelter, tenting, and trail locations that northbound through-hikers encounter on the AT.
Materials and methods
We used a stratified random sample approach to survey 42 shelter areas on a south-to-north transect along the entire Appalachian Trail (table 1) in months coinciding with peak nymphal tick activity, when Lyme disease risk is considered highest (Diuk-Wasser et al. 2006). We used a 0.5 m ² (5 ft²) white cloth flag to sweep surfaces and vegetation to collect ticks (AFPMB 2012). At each site, we flag-swept three areas: (1) shelter floors, steps, and low walls; (2) a 1,000 m² (1,196 yd²) tenting area surrounding the shelter; and (3) a 500-meter-long by half-meter-wide (1,640 ft × 1.6 ft) linear swath of trail. Collected ticks were placed in zipper-style plastic bags and shipped to the U.S. Army Public Health Command, Aberdeen Proving Ground, Maryland, for species identification and pathogen detection (using standard polymerase chain reaction [PCR] techniques described in Han et al. 2014). We tested all ticks collected for associated human pathogens.
| Table 1. Tick and pathogen collections along the Appalachian Trail, 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Shelter Site¹ | Date | State² | Elevation (m) | Tick Count | Species³ (Number Collected) |
Pathogen Status | ||
| Shelter | Tent Camp | Trail | ||||||
| Stover Creek | 13-Apr | Ga. | 875 | 0 | 0 | 0 | ||
| Hawk Mountain | 13-Apr | Ga. | 975 | 0 | 0 | 0 | ||
| Long Branch | 20-Apr | NT | 1,352 | 0 | 0 | 0 | ||
| Mollies Ridge | 25-Apr | NT | 1,393 | 0 | 0 | 0 | ||
| Silers Bald | 26-Apr | NT | 1,664 | 0 | 0 | 0 | ||
| Overmountain | 10-May | NT | 1,387 | 0 | 0 | 0 | ||
| Watauga Lake | 13-May | NT | 649 | 0 | 0 | 0 | ||
| Abingdon | 14-May | NT | 1,154 | 0 | 0 | 0 | ||
| Knot Maul Branch | 21-May | Va. | 878 | 0 | 0 | 0 | ||
| Pine Swamp Branch | 27-May | Va. | 771 | 0 | 0 | 0 | ||
| Laurel Creek | 29-May | Va. | 829 | 0 | 0 | 2 | Dv (2) | 0 |
| Bryant Ridge | 3-Jun | Va. | 402 | 0 | 0 | 3 | Aa (3) | 0 |
| Brown Mountain Creek | 5-Jun | Va. | 425 | 0 | 0 | 2 | Dv (1), Aa (1) | 0 |
| Blackrock Hut | 14-Jun | Va. | 806 | 0 | 0 | 18 | Aa (16), Am (1), Dv (1) | Rm-1 |
| Crampton Gap | 21-Jun | Md. | 305 | 0 | 0 | 0 | ||
| Raven Rock | 22-Jun | Md. | 451 | 0 | 0 | 0 | ||
| Raush Gap | 29-Jun | Pa. | 296 | 0 | 0 | 1 | Is (1) | Bb |
| Windsor Furnace | 2-Jul | Pa. | 268 | 0 | 0 | 0 | ||
| George W. Outerbridge | 4-Jul | Pa. | 305 | 0 | 0 | 0 | ||
| Kirkridge | 6-Jul | Pa. | 451 | 0 | 0 | 0 | ||
| Mashipacong | 8-Jul | N.J. | 434 | 0 | 1 | 1 | Dv (1) | 0 |
| Rutherford | 8-Jul | N.J. | 410 | 1 | 0 | 0 | Dv (1) | 0 |
| Pochuck Mountain | 9-Jul | N.J. | 256 | 1 | 1 | 0 | Dv (1) | 0 |
| Fingerboard | 11-Jul | N.Y. | 396 | 0 | 0 | 0 | ||
| William Brien Memorial | 12-Jul | N.Y. | 326 | 0 | 0 | 0 | ||
| Wiley | 16-Jul | N.Y. | 226 | 0 | 0 | 0 | ||
| Limestone Spring | 19-Jul | Conn. | 299 | 0 | 0 | 0 | ||
| Riga Leanto | 20-Jul | Conn. | 491 | 0 | 0 | 0 | ||
| Mt. Wilcox S | 21-Jul | Mass. | 524 | 0 | 0 | 2 | Is (2) | 0 |
| Mt. Wilcox N | 21-Jul | Mass. | 594 | 0 | 0 | 0 | ||
| October Mountain | 22-Jul | Mass. | 588 | 0 | 0 | 0 | ||
| Spruce Peak | 27-Jul | Vt. | 664 | 0 | 0 | 0 | ||
| Greenwall | 29-Jul | Vt. | 617 | 0 | 0 | 0 | ||
| Clarendon | 30-Jul | Vt. | 363 | 0 | 0 | 0 | ||
| Stoney Brook | 31-Jul | Vt. | 536 | 0 | 0 | 0 | ||
| Hexacuba | 5-Aug | N.H. | 604 | 0 | 0 | 0 | ||
| Ethan Pond | 11-Aug | N.H. | 899 | 0 | 0 | 0 | ||
| Rattle River | 16-Aug | N.H. | 384 | 0 | 0 | 0 | ||
| Frye Notch | 17-Aug | Maine | 695 | 0 | 0 | 0 | ||
| Bemis Mountain | 19-Aug | Maine | 850 | 0 | 0 | 0 | ||
| Sabbath Day Pond | 20-Aug | Maine | 728 | 0 | 0 | 0 | ||
| Wilson Valley | 29-Aug | Maine | 296 | 0 | 0 | 0 | ||
| All sites | 2 | 2 | 29 | 2 | ||||
| ¹GPS coordinates can be found at http://web.eecs.utk.edu/~dunigan/at/. ²“NT” indicates the border of North Carolina and Tennessee. ³Species abbreviations: Aa: Amblyomma americanum, Am: Amblyomma maculatum, Dv: Dermacentor variabilis, Is: Ixodes scapularis, Rm: Rickettsia montanensis, Bb: Borrelia burgdorferi |
||||||||
Results
We conducted a total of 126 flagging sessions (i.e., three areas each at 42 shelters), of which 11 (8.7%) produced ticks (table 1). The map shows the location of shelter areas where ticks were collected (see fig. 1). Despite sampling, we collected no ticks from Georgia, North Carolina, Tennessee, Maryland, New York, Connecticut, Vermont, New Hampshire, or Maine. Sampling extended from mid-April through August, but ticks were collected only from 29 May to 21 July (table 1).
One notable collection was of A. americanum and A. maculatum in western Albemarle County, Virginia, at 806 m (2,644 ft) in elevation; this is the first record of the latter species in Albemarle County. B. burgdorferi and Rickettsia montanensis (one infected tick each) were the only human pathogens detected among the 33 ticks tested.
Discussion
We conducted trail sampling in a linear swath of trail 0.5 m (1.6 ft) wide. Entomologists report tick density per 1,000 m2. Overall tick abundance on the trail was low (2.8 ticks per 1,000 m2 of trail), which is consistent with low densities recorded by Diuk-Wasser et al. (2006) in the Appalachian Mountains. Ticks were rarely found around shelters or tenting areas, and were 14.5 times more likely to be encountered on the trail itself. Low tick abundance is likely a consequence of the high elevation of many segments of the trail; the trail averages 760 m (2,493 ft) in elevation overall and no ticks were collected above 830 m (2,723 ft). All ticks collected came from central Virginia, Pennsylvania, New Jersey, or Massachusetts, where the trail is generally lower than the approximately 500 m (1,600 ft) limit for I. scapularis noted by Bunnell et al. (2003). The high elevations of the Appalachian Trail, combined with subalpine coniferous forest and alpine vegetation, constitute poor tick habitat (Jouda et al. 2004), which helps explain why no ticks were collected in Vermont, New Hampshire, or Maine despite efforts to survey these areas.
Season is an important determinant of hiker exposure to ticks. The lack of springtime collections in the southern states reflects low spring temperatures (and high elevations) of the trail, although repeat surveys in other seasons will be needed to properly quantify such patterns. The importance of the seasonally restricted sampling we report here is that it coincides with the typical timing of most hikers’ and maintenance crews’ use of the AT.
This survey provides only limited information on the pathogen status of the ticks, as sample sizes were small. One of three I. scapularis was infected with B. burgdorferi, which is consistent with the >20% prevalence of this pathogen typically found in nymphs in Lyme disease–endemic areas (Stromdahl and Hickling 2012). Prevalence of pathogenic Ehrlichia and Rickettsia species is low in human-biting ticks (typically <10%; Stromdahl and Hickling 2012), so it is unsurprising that only a single Rickettsia species was detected in our sample.
Tick prevention measures

Karl Ford
Season, habitat type, and elevation are determinants of the risk of hiker exposure to ticks on the Appalachian Trail. This research indicates the greatest exposure to deer ticks occurs from May to July in the states of Maryland, Pennsylvania, New Jersey, New York, Connecticut, and Massachusetts, and at elevations less than around 500 meters (1,600 ft). Because of the high incidence and severity of the disease and concern for the health problems that can result, hikers should be aware of tick-prone areas, symptoms of the disease, and methods for minimizing contact with ticks. The National Park Service and other land management agencies could consider messaging as a way to provide information to prospective hikers to help make hiking safer.
The Centers for Disease Control and Prevention makes several recommendations to reduce the chance of tick bites:
- Wear factory-treated permethrin clothing and treat shoes, pack, and outer tent floor with spray-on 0.5% permethrin (http://www.cdc.gov/lyme/prev/on_people.html). Permethrin binds to clothing, is safe for humans, and is highly repellent of ticks, spiders, and insects (http://www.epa.gov/pesticides/factsheets/factory-treated-clothing.html). We recommend treated ventilating bug-net pants, long-sleeved shirt, and hat. Proper clothing treated with permethrin is the single most important preventive measure a hiker can take.
- Apply 20–30% DEET repellent on exposed skin, but use it sparingly and according to directions on adults and children because DEET is toxic. Higher concentrations are unnecessary. Alternatively, wear a net to protect your head and face.
- Conduct a daily (or more frequent) tick check. Look for adult or juvenile (nymph) ticks (see fig. 3). Enlist a hiking buddy to check your back. Perform a partial check of body and clothing during breaks and after collecting firewood and do a more complete check at the end of the day.
- Shower or bathe as soon as possible after leaving the trail and conduct a full-body tick check. Put clothing in a dryer for an hour to kill any ticks present.
- Be able to identify deer ticks and recognize the symptoms of Lyme disease (http://www.cdc.gov/lyme/ or http://www.lymediseaseassociation.org).
- Immediately remove any attached tick with forceps with a slow, steady pull. Wash the bite area carefully with soap and water or sanitizer.
- Seek medical treatment immediately if any flulike symptoms occur and be aware that an expanding rash may or may not be present.
The lead author hiked the entire trail and has several additional recommendations for avoiding contact with ticks:
- Hike the center of the trail and use caution hiking off-trail, such as when collecting firewood. Tick exposure is reduced by regular trail, field, power line, and shelter area vegetation trimming (fig. 4).
- Avoid sitting on the ground or on logs and refrain from setting your pack or gear on the ground, if possible. Treat a closed-cell sit pad with permethrin for rest breaks.
- Avoid pitching your tent in leaf piles or grass. Tents may be safer than tarps. Bare ground is safer than areas covered with leaf litter or grass and is in accordance with Leave-No-Trace principles. Shelters and picnic tables are fairly safe places, but examine them first.
- Avoid hiking and camping with dogs, as dogs attract ticks. Close contact, as in petting or sleeping, may enable the ticks to bite humans.
Further research
Although this work was the first study of tick abundance encompassing the entire AT, it is limited to the dates and locations sampled. Additional trailside sampling is needed in the southern states and in Maine from May to July. In addition, more intensive trailside sampling from northern Virginia through Connecticut could better identify high-risk areas. Modeling and observational data suggest I. scapularis is extending its range because of climate change (Ogden et al. 2014).
Acknowledgements
JoAnne Oliver, New York State Department of Health, Albany, and David Wong, National Park Service, Albuquerque, New Mexico, provided advice during the study. Joyce Kopatch, Mary Vince, and Chad Elkins, U.S. Army Public Health Command, Aberdeen Proving Ground, Maryland, provided technical assistance. The Lyme Disease Association, Jackson, New Jersey, provided financial support. The National Park Service provided research access to the AT and support to the project.
References
AFPMB. 2012. Tick-borne diseases: Vector surveillance and control. Technical Guide No. 26. Armed Forces Pest Management Board, Department of Defense, Silver Spring, Maryland, USA.
Bunnell, J. E., S. D. Price, A. Das, T. M. Shields, and G. E. Glass. 2003. Geographic information systems and spatial analysis of adult Ixodes scapularis (Acari: Ixodidae) in the Middle Atlantic region of the U.S.A. Journal of Medical Entomology 40:570–576.
Centers for Disease Control and Prevention (CDC). 2013. Reported cases of Lyme disease by state or locality, 2003–2012. Accessed 4 August 2014 at http://www.cdc.gov/lyme/stats/chartstables/reportedcases_statelocality.html.
Diuk-Wasser, M. A., A. G. Gatewood, M. R. Cortinas, S. Roberto, S. Yaremych-Hamer, J. Tsao, U. Kitron, G. Hickling, J. S. Brownstein, E. Walker, J. Piesman, and D. Fish. 2006. Spatiotemporal patterns of host-seeking Ixodes scapularis nymphs (Acari: Ixodidae) in the United States. Journal of Medical Entomology 43:166–176.
Han, G., E. Stromdahl, D. Wong, and A. Weltman. 2014. Exposure to Borrelia burgdorferi and other tick-borne pathogens in Gettysburg National Military Park, south-central Pennsylvania, 2009. Vector-Borne Zoonotic Disease 14(4):227–233.
Jouda, F., J. L. Perret, and L. Gern. 2004. Ixodes ricinus density, and distribution and prevalence ofBorrelia burgdorferi sensu lato infection along an altitudinal gradient. Journal of Medical Entomology 41:162–169.
Knoll, J. M., A. C. Ridgeway, C. M. Boogaerts, and G. A. Burket III. 2014. Appalachian Trail hikers’ ability to recognize Lyme disease by visual stimulus photographs. Wilderness and Environmental Medicine 25(1):24–28.
Mead, P. 2013. Estimating the public health burden of Lyme disease in the United States. Presented 19 August 2013 at the 13th International Conference on Lyme Borreliosis and Other Tick-Borne Diseases, Boston, Massachusetts, USA.
Ogden, N. H., M. Radojevic, X. Wu, V. R. Duwari, P. A. Leighton, and J. Wu. 2014. Estimated effects of projected climate change on the basic reproductive number of the Lyme disease vector Ixodes scapularis. Environmental Health Perspectives 122(6):631–638. doi:10.1289/ehp.1307799.
Oliver, J., and J. J. Howard. 1998. Occurrence of Ixodes scapularis along a selected segment of the Appalachian Trail. Journal of Medical Entomology 35:54–58.
Stromdahl, E., and G. Hickling. 2012. Beyond Lyme: Aetiology of tick-borne human diseases with emphasis on the southeastern United States. Zoonoses Public Health 59 (Suppl. 2):48–64.
About the authors
Karl Ford is an environmental scientist and natural resource specialist, retired from the Bureau of Land Management. He lives in Golden, Colorado, and can be reached by e-mail. Robyn Nadolnyis a PhD candidate in ecological sciences in the Department of Biological Sciences, Old Dominion University, Norfolk, Virginia. Ellen Stromdahl is an entomologist with the U.S. Army Public Health Command, Aberdeen Proving Ground, Maryland. Graham Hickling is a research associate professor and director of the Center for Wildlife Health, Department of Forestry, Wildlife and Fisheries, University of Tennessee in Knoxville.
Documentation
Suggested citation for this article
Ford, K. R. Nadolny, E. Stromdahl, and G. Hickling. 2015. Tick surveillance and disease prevention on the Appalachian Trail. Park Science 32(1):36–41.
This article published
Online: 4 September 2015; In print: 14 September 2015
URL
https://www.nps.gov/articles/parkscience32_1_36-41_ford_et_al_3819.htm
This page updated
16 September 2015
Site navigation
Last updated: August 9, 2018
