Lake Lucero is a highly saline and seasonally aquatic playa; it is the source of the White Sands National Monument’s gypsum dunes of the Tularosa Basin in Southern New Mexico.

© Adrian Unc
The surface of the Lake Lucero playa is covered with patches of halite (rock salt) and tends to have a shallow clay soil profile of no more than 25 cm (10 in) underlied by compact coarse gypsum mixed with larger selenite crystals (a form of gypsum and the source of the White Sands). Despite its location at the northern tip of the Chihuahuan Desert the lakebed is constantly wet due to a shallow groundwater table, no more than 100 cm (39 in) under its surface. It is also reported that hot groundwater might discharge into the lake. This water is reported to be more acidic and to favor extreme thermophilic organisms (microorganisms adapted to high temperatures).
This combination of an acidic hot groundwater and alkaline, highly saline soil profile raises interesting questions on the genetic diversity of the soil microorganisms and their associated metabolic functions, especially their distribution with soil depth.
Methods
|
Research Objective: To assess microbial genetic and functional diversity in the soils of the playa of Lake Lucero. |
Sample Site
We collected samples from three representative lakebed soil profiles near the western shore of Lake Lucero.
The top 25 cm (10 in) of soil were sampled, as the large concentration of compacted gypsum sand and selenite minerals did not permit any deeper excavation. The very thin soil crust and five soil horizons were sampled for microbial genetic diversity analyses.
Microbial Diversity
Microbial diversity was assessed through sequencing of a universal taxonomic marker gene; three different segments of the same gene were considered for bacterial diversity. The methods are named by the starting primer employed to isolate and sequence them from the total extracted DNA: 27f (Method 1), 699f (Method 2), and 106f (Method 3). Fungal diversity was estimated through the variability of a fungal specific taxonomic marker genetic sequence (endoITS). Both bacterial and fungal indicator sequences are part of the ribosomal DNA, a common target for molecular systematics.
Soil Community Physiological Profiling (CLPP)
Five soil layers were sampled for the CLPP tests. Functional diversity was assessed through estimates of the capability of the soils to utilize certain single carbon source substrates (see box). A surfactant, “Tween,” known to enhance enzymatic activities in soils was used as another substrate.
Substrates for CLLP |
|
|
Amino acids Carboxylic acids |
Carbohydrates Polysacharides Other |
Results
Microbial Diversity
The methods used to describe bacterial diversity did produce slightly different taxonomic results. Methods 1 and 3 were effective at describing bacterial diversity; however, method 2 also described the diversity of Archaea (evolutionarily older unicellular organisms with no cell nucleus or any other membrane-bound organelles within their cells) and bacteria adapted to extreme conditions.
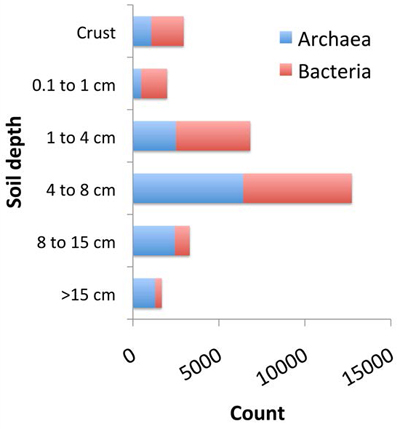
Diversity increased with distance from the surface being greatest in the 4 cm to 8 cm (~1.6 to 3.15 in) horizon to only decline for the deeper soil layers (Figure 1). Bacteria dominated the superficial soil layers, while Archaea were dominant at depth.
While thermophilic organisms, (that prefer temperatures greater than 45°C [113°F]) could be identified at all depths (Figure 2), their proportion at different depths was clearly linked to the decrease in temperature away from the soil surface. About 75% of all Bacteria and Archaea in the soil crust had an optimal growth temperature of over 85°C (185°F). Most soil-crust organisms are temperature, radiation, and salinity resistant; however, more common microorganisms, and even some known to colonize vascular plants, were also found.
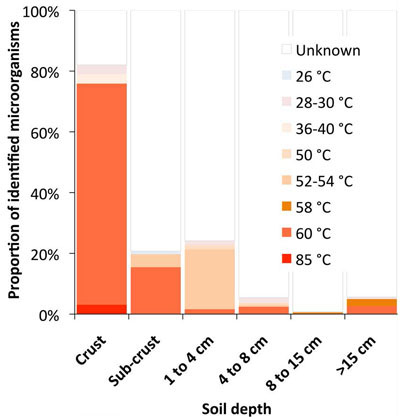
Archaea seem to be in charge of the fixation of atmospheric nitrogen and the production of nitrates.
Some blue-green algae have been identified at 4 cm to 8 cm (~1.6 to 3.15 in) depth suggesting that this layer might act as storage compartment for algae during the dry season.
Functionality, Soil Community-level Physiological Profiling
Figure 3 summarizes the potential of the different soil layers to break down the substrates presented above (results are expressed as the proportion of respired CO2 by the biomass in the respective soil layer). The 1st, 3rd, and 5th soil layers have the potential to degrade all tested substrate.
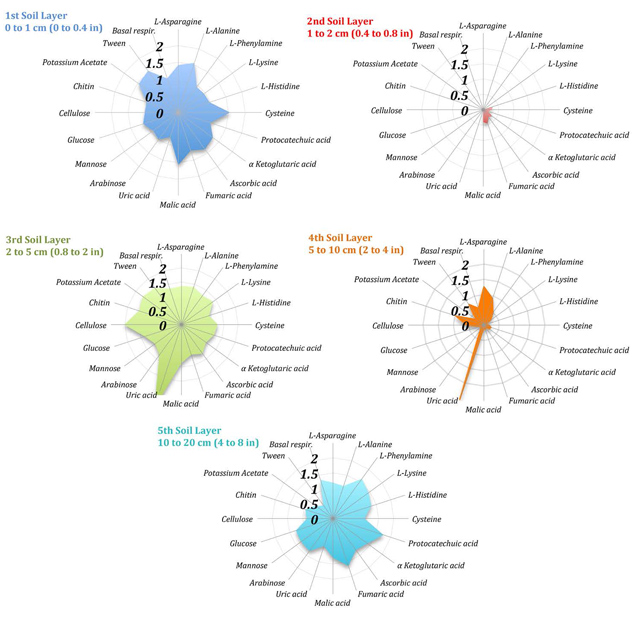
Urea is very rapidly consumed in the 3rd and 4th soil layers; possibly with the participation of Archaea. The same soil layers are also rich in fungi able to consume complex organic compounds such as chitin and cellulose. The subcrust soil layer (2nd) is rather inactive.
These metabolic activities have been measured at 22°C to 24°C (72°F to 75°F), indicating that despite the large dominance of thermophiles, the soil also harbors active organisms that grow best at more moderate temperature, neither too hot nor too cold (mesophiles), that maintain their activity potential during the warmer seasons and become active during the cooler seasons.
Conclusions
Lake Lucero harbors unique microbial communities whose diversities are governed by the temperature gradients in the soil profiles. The topsoil crust harbors organisms resistant to extreme heat and chemical conditions. Profiling of the potential physiological activity suggests that complex enzymatic mechanisms are present and available over the whole depth of the tested soil profile. Therefore, both diversity and functional potential vary by soil layer. Soil layers harboring highly diverse microbial populations with high functional capabilities are separated from each other by soil layers with lower diversity and more limited functional capabilities. This discontinuous distribution with depth is likely due to the historical layering and seasonal changes in the direction of the water movement as wet and dry seasons alternate.
It is clearly of interest to identify how and if the same microbial consortia are distributed across the gypsum dunes with the associated distribution of their functional patterns.
For more information, contact:
Adrian Unc, aunc@nmsu.edu
Collaborators
Adrian Unc, Curtis Monger, Mary Lucero, and Conrad Nelson
Plant and Environmental Sciences
New Mexico State University
Hildy Reiser, David Bustos, and Pete Biggam
National Park Service
Prepared in collaboration with the National Park Service, 2012.
Last updated: November 30, 2016
