Part of a series of articles titled Yellowstone Science - Volume 27 Issue 1: Vital Signs - Monitoring Yellowstone's Ecosystem Health.
Article
SHORT: Aquatic Vascular Macrophytes as Vital Signs

Photo: C. Eric Hellquist.
Aquatic Vascular Macrophytes as Vital Signs: Ecological Importance & Management Considerations for the GYE
by C. Eric Hellquist, C. Barre Hellquist, and Heidi M. Anderson
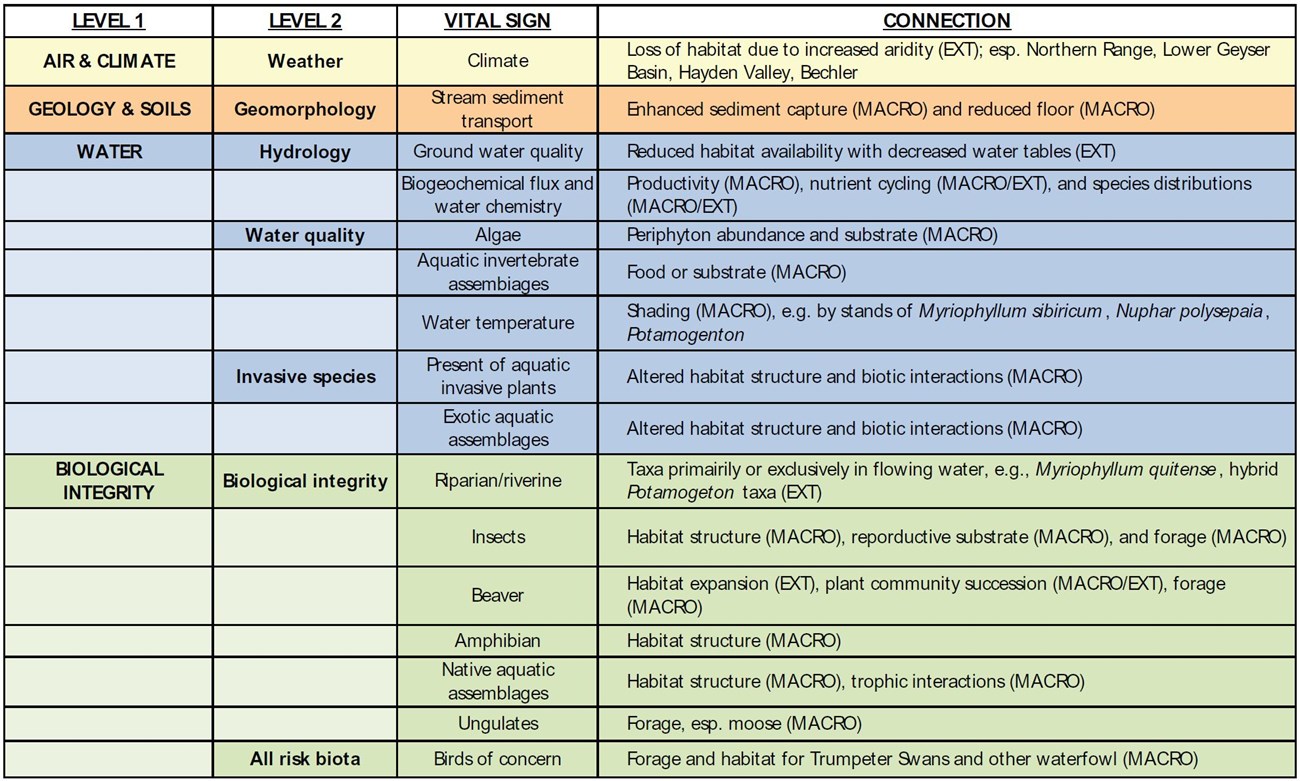
Large, readily visible plants (macrophytes) are central species of aquatic ecosystems. Macrophytes have diverse morphological and ecological strategies for living in divergent ecological conditions or niches that span the water column. For example, macrophytes can be free-floating on the surface, entirely or partially submerged, and emergent. Of the 41 vital signs selected for Yellowstone National Park (YNP), nearly two of every five (40%) can be connected to macrophytes (table 1; Jean et al. 2005).
Macrophytes respond to external conditions (e.g., climate, hydrology, and site productivity) yet also create conditions that define habitats for other biota (table 1). Waterfowl (Squires and Anderson 1995) and some mammals (McMillan 1953) use macrophytes as important food sources. Macrophytes are substrate for algae and other microscopic organisms (Pip and Robinson 1984) and provide habitat for fish nesting, egg attachment, and shelter (Dibble et al. 1996). Like amphibians (e.g., McMenamin et al. 2008), macrophytes also can provide clues of distressed populations and habitats.
Since 2008, the macrophyte diversity of YNP and Grand Teton National Park (GTNP) has been inventoried (Hellquist et al. 2014). Comprehensive field surveys have located more than 90 species of macrophytes in YNP and over 70 species in GTNP. Over 2,100 unique specimens have been obtained from approximately 390 field sites in YNP, GTNP, and the National Elk Refuge near Jackson, WY. Many collections are range expansions and new records for the Greater Yellowstone Ecosystem (GYE; Hellquist et al. 2014). These records document aquatic communities ahead of exotic introductions (Westbrooks 2004) and serve as mileposts of succession as wetland and aquatic habitats contract in a projected drier future for the GYE.
As of 2016-2017, no non-native, entirely aquatic invasive macrophytes (Roper 2017, Hellquist et al. 2014) have been detected in YNP or GTNP; however, three exotic wetland species (Nasturtium officinale W. T. Aiton, Myosotis scorpioides L., and Mentha spicata L.) were observed. Invasive macrophytes are abundant throughout the lower 48 states and easily spread by people. The absence of invasive macrophytes in YNP and GTNP is remarkable. Their absence in YNP and GTNP is due in large part to the vigilance of National Park Service boat inspections and good fortune. In 2017, more than 3,500 vessels from 47 U.S. states were inspected in YNP alone (Roper 2017). Fifty-six vessels were decontaminated, several of which carried macrophytes. An added benefit of boat inspections was educational outreach to approximately 10,400 visitors (Roper 2017).
Boat inspections are crucial for intercepting human-mediated introductions, but aquatic invasive species (AIS) can arrive via animals as well. Waterfowl regularly disperse macrophyte vegetative structures or propagules (Brochet et al. 2009) and may introduce AIS from waters outside of the parks. Continued monitoring and an established rapid response plan will be critical to address the arrival of AIS. A proactive plan would outline protocols (e.g., waterfowl or wildlife surveys, containment standards) so action can be taken if a nascent invasion is discovered. Surveys should be concentrated in areas with the most boat use and fishing activity and where migratory waterfowl congregate. Ideally, these surveys would be coordinated with ongoing fisheries and amphibian monitoring efforts at multiple, overlapping sites.
Low temperature hydrothermal areas are also a potential habitat of concern for AIS and merit consideration for regular monitoring. Some hydrothermal habitats could provide refugia for invasive southern taxa or unique genetic forms (i.e., genotypes). For example, an unusual population of the macrophyte Najas guadaloupensis has been long established in Kelly Warm Springs, GTNP. Based on the abundance of exotic aquarium fish at Kelly Warm Springs (Harper and Farag 2017) and the unusual genetic identity of this Najas population, it’s highly probable that the Najas population may have originated from discarded aquarium plants.
An emerging management tool that provides promising opportunities is environmental DNA (eDNA). Isolated from water samples, eDNA can provide evidence of invasive macrophyte colonization and potentially the relative biomass of populations (Matsuhashi et al. 2016). Genetic signatures of high-risk invasive macrophytes such as Eurasian water milfoil (Myriophyllum spicatum L.) could be targeted. The use of eDNA could be a time and cost efficient method to detect an unfolding invasion.
While preventing invasive macrophytes from entering YNP requires a commitment of time, funds, and personnel, it is a wholly worthwhile investment (Leung et al. 2002, Westbrooks 2004). Proactive efforts are less financially and logistically expensive than trying to manage macrophyte invaders once they colonize. Because asexual propagation of most invasive macrophytes makes eradication unlikely, early detection is essential. Once established, treatment options become complicated by ecological, logistical, economic, and political concerns.
Additional funding for invasive macrophyte monitoring and management could originate from a number of sources, such as park entrance fee revenue or recreational permit fees. A more extreme measure could be suspension of motorized boat use by visitors. Alternatively, visitors could be restricted to using watercraft that are overseen by the park and do not leave YNP waters. These options would be controversial, but would reduce a major introduction pathway for invasive macrophytes and other AIS. If employed, resources formerly used for boat inspections could be reallocated to macrophyte field surveys and regular monitoring.
The aquatic and wetland habitats in the GYE contain diverse native macrophyte assemblages. These plants are important for their foundational role as the basis of the food chain and their extensive ecological interactions. Meanwhile, the potential for invasive macrophytes to enter the GYE is a looming, formidable concern for the conservation and management of regional aquatic ecosystems. Continued research and monitoring will improve our understanding of the role and importance of macrophytes in aquatic ecosystems and will alert park managers to the unwelcome potential arrival of invasive species.
Literature Cited
Abrochet, A-L., M. Guillemain, H. Fritz, M. Gauthier-Clerc, and A.J. Green. 2009. The role of migratory ducks in the long-distance dispersal of native plants and the spread of exotic plants in Europe. Ecography 32:919-928.
Dibble, E.D., K.J. Kilgore, and S.L. Harrel. 1996. Assessment of fish-plant interactions. American Fisheries Society Symposium 16:357-372.
Harper, D.D., and A.M. Farag. 2017. The thermal regime and species composition of fish and invertebrates in Kelly Warm Spring, Grand Teton National Park, Wyoming. Western North American Naturalist 77:440-449.
Hellquist, C.E., C.B. Hellquist, and J.J. Whipple. 2014. New records for rare and under-collected aquatic vascular plants of Yellowstone National Park. Madroño 61:159-176.
Jean, C., A.M. Schrag, R.E. Bennetts, R. Daley, E.A. Crowe, and S. O’Ney. 2005. Vital signs monitoring plan for the Greater Yellowstone Network. National Park Service, Greater Yellowstone Network, Bozeman Montana, USA.
Leung, B., D.M. Lodge, D. Finnoff, J.F. Shogren, M.A. Lewis, and G. Lamberti. 2002. An ounce of prevention or a pound of cure: bioeconomic risk analysis of invasive species. Proceedings of the Royal Society London B 269:2407-2413.
Matsuhashi, S, H. Doi, A. Fujiwara, S. Watanabe, and T. Minamoto. 2016. Evaluation of the environmental DNA method for estimating distribution and biomass of submerged aquatic plants. PLoS ONE 11(6):e0156217.
McMenamin, S.K., E.A. Hadly, and C.K. Wright. 2008. Climatic change and wetland dessication cause amphibian decline in Yellowstone National Park. Proceedings of the National Academy of Sciences USA 105:16988-16993.
McMillan, J.F. 1953. Some feeding habits of moose in Yellowstone Park. Ecology 34:102-110.
Pip, E., and G.G.C. Robinson. 1984. A comparison of algal periphyton composition on eleven species of submerged macrophytes. Hydrobiological Bulletin 18:109-118.
Roper, J. 2017. Aquatic invasive species (AIS) 2017 prevention report, December 27, 2017. Resource Management Operations. National Park Service, Yellowstone National Park, Mammoth, Wyoming, USA.
Squires, J.R., and S.H. Anderson 1995. Trumpeter Swan (Cygnus buccinator) food habits in the Greater Yellowstone Ecosystem. American Midland Naturalist 133:274-282.
Westbrooks, R. 2004. New approaches for early detection and rapid response to invasive plants in the United States. Weed Technology 18:1468-1471.
C. Eric Hellquist is an Associate Professor of Biological Sciences at the State University of New York at Oswego. As a plant ecologist, his central research interests concern plant interactions in aquatic and wetland ecosystems. Eric has worked in Yellowstone since 2006. His Greater Yellowstone research is focused on the ecology of aquatic vascular plants in Yellowstone and Grand Teton national parks.
C. Barre Hellquist, Professor Emeritus at Massachusetts College of Liberal Arts, has been studying the ecology and systematics of aquatic plants for over 45 years. Much of his research has focused on pondweeds (Potamogetonaceae) and water-lilies (Nymphaeaceae). His field studies have been concentrated in North America, Australia, and Russia. He and his son Eric have been documenting the aquatic plant diversity of the Greater Yellowstone Ecosystem since 2008.
Last updated: September 23, 2019
