Part of a series of articles titled Alaska Park Science - Volume 17, Issue 1. Migration: On the Move in Alaska.
Article
Influence of Spring Prey Pulses on Seasonal Migrations of Pinnipeds in the North Pacific Ocean

Central to understanding the migration of organisms is an understanding of why they move. Explanations for migratory behavior may include reducing the risk of predation, enhancing access to breeding opportunities, or enhancing access to aggregations of high-quality food or shifting patterns of food abundance (Alerstam et al. 2003, Milner-Gulland et al. 2011).
Each spring in the marine waters of the northeast Pacific Ocean, spawning aggregations of forage fish provide an episodic influx of energy to coastal and nearshore regions that cascades throughout marine ecosystems. The spawning aggregations of forage fish are an example of a resource pulse (e.g., Yang et al. 2010) that provides a “moveable feast” and short-term burst of prey availability for numerous invertebrates, fish, seabirds, and marine mammals (Willson and Womble 2006). The predictable prey pulse provided by spawning aggregations of forage fish including eulachon (Thaliechthys pacificus), capelin (Mallotus villosus), and herring (Clupea pallasii), supplies a significant source of calories (e.g., Surma et al. 2018) prior to the energetically demanding pupping and breeding season of pinnipeds, including Steller sea lions (Eumetopias jubatus) and harbor seals (Phoca vitulina richardii; Womble et al. 2005). These marine mammals inhabit the nearshore coastal waters of the northeast Pacific Ocean and the marine waters in and adjacent to Alaska’s parklands. Changes in the distribution and seasonal migrations of these pinnipeds have been documented and linked to the availability of high-quality prey that varies both temporally and spatially (Womble et al. 2008, Womble and Gende 2013, Sigler et al. 2017).
The annual cycle of pinnipeds is composed of reproductive, non-breeding, and migration periods that may vary in timing and location. Although periods of the annual cycle may be separated geographically by hundreds or thousands of miles, they are fundamentally linked as conditions encountered during the non-breeding season may directly influence the reproductive season (e.g., Marra et al. 2015). This is particularly true for capital breeders, those species that rely primarily upon stored energy reserves acquired prior to the reproductive season to fuel energy costs associated with reproduction and other critical life-history phases (Jönsson 1997). Most phocids, (Family Phocidae comprised of true seals) including harbor seals, have largely separated feeding from reproduction (Costa 1991) and rely primarily on stored energy to fuel the brief lactation period that typically ranges from 4-45 days (Bowen et al. 1985, Oftedal et al. 1987). For example, the extensive migrations of northern elephant seals (Mirounga angustirostris) from California to the mesopelagic zone of the northeast Pacific Ocean demonstrate the extreme separation between pupping/breeding sites and foraging areas (LeBoeuf et al. 2000, Robinson et al. 2012). In contrast, the otariids (Family Otariidae comprised of sea lions and fur seals) are income breeders and rely upon intermittent foraging throughout an extended lactation period during which adult females provision their young, and are dependent upon local prey availability (Boyd 1998).
The seasonal energy pulse provided by spring aggregations of forage fish undoubtedly provides a significant energy influx for pinnipeds prior to the pupping and breeding season with likely implications for reproduction and survival for both capital and income breeders. Numerical responses, shifts in distribution, and shifts in the diet of pinnipeds provide evidence that the influx of the seasonal prey aggregations is important for both harbor seals and Steller sea lions (Sigler et al. 2004, Womble et al. 2005, Womble and Gende 2013). Herein, examples of seasonal migrations of harbor seals and Steller sea lions are described in relation to the influx of spawning aggregations of forage fish in and adjacent to the marine waters of Alaska’s national parklands.
Harbor seals are primarily capital breeders and are the most widely distributed pinniped in the northern hemisphere. In Alaska, harbor seals come ashore at terrestrial sites and also use icebergs in tidewater glacier fjords to pup, breed, and molt (Mathews and Pendleton 2006, Womble et al. 2010). Tidewater glacier fjords host some of the largest seasonal aggregations of harbor seals in Alaska; however, a satellite telemetry study conducted in Glacier Bay National Park and Preserve demonstrates that harbor seals travel widely outside of the reproductive season (from September to April) with some harbor seals migrating over 560 miles (900 kilometers) away. Juvenile and adult female harbor seals traveled extensively both within Glacier Bay and throughout much of northern southeast Alaska, the eastern Gulf of Alaska, Prince William Sound, and to other tidewater glacier habitats in Disenchantment and Icy bays adjacent to Wrangell-St. Elias National Park and Preserve. Harbor seals departed Johns Hopkins Inlet, the primary glacier ice site in Glacier Bay, between September and November and began to return the following year in April. Although harbor seals traveled widely during the post-breeding season, they exhibited a high degree of fidelity back to Glacier Bay the following pupping season (May-June; Womble and Gende 2013).
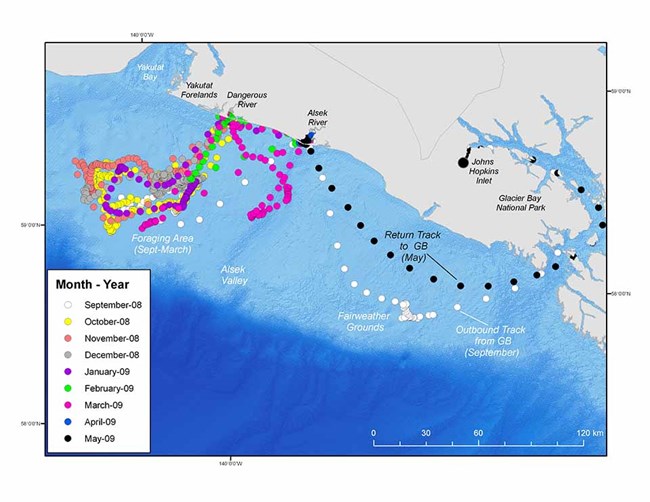
There was a high degree of individual variability in post-breeding season migration patterns of harbor seals from Glacier Bay and several lines of evidence suggest that some harbor seals migrated to take advantage of seasonal aggregations of forage fish. For example, one adult female seal (PV08GB21) traveled from Johns Hopkins Inlet in Glacier Bay and spent >200 days in the eastern Gulf of Alaska and made several extended forays to an area near the continental shelf edge approximately 60 miles (95 km) offshore. Beginning in February through mid-May, the adult female seal moved to the mouth of the Alsek River, near Dry Bay, where eulachon, an energy-rich forage fish, spawns during spring. In mid-May, the adult female seal traveled from the Alsek River back to Johns Hopkins Inlet in Glacier Bay where she was tagged the previous autumn (Womble and Gende 2013; Figure 1). Aggregations of harbor seals have also been observed hauled out (>900 seals) and foraging in the Alsek River (Figure 2; Jamie Womble, personal observation, April 2013). Similarly, three harbor seals from Glacier Bay also traveled to estuaries in Chilkat Inlet and Lutak Inlet in northern Lynn Canal when eulachon, capelin, and herring were spawning.

NPS/Jamie Womble
The distributions and seasonal migrations of Steller sea lions are also influenced by the availability of spring-spawning forage fish in many regions throughout Alaska in and adjacent to Alaska parklands. Over 2,000 Steller sea lions regularly aggregate at Dry Bay near the mouth of the Alsek River, along the border of Glacier Bay National Park and Preserve (Figure 3) and at several other rivers along the Yakutat Forelands during spring when eulachon are spawning (Womble et al. 2008, Mathews et al. 2011). Steller sea lions from haulouts and rookeries throughout southeast Alaska make seasonal migrations to the Alsek River during spring to take advantage of this high energy, densely aggregated prey resource. In fact, several Steller sea lions that were marked in southeastern Alaska have been observed at Dry Bay (Rehberg et al. In revision).

NPS/Jamie Womble
Steller sea lions also aggregate in response to spring-spawning fish in other areas in and near Glacier Bay National Park and Preserve including Adams Inlet, Tarr Inlet, and the estuaries and lower reaches of the Dixon River and the Excursion River. Steller sea lions respond to spring-spawning aggregations of eulachon in the estuary at the mouth of the Taiya River near Klondike Goldrush National Historical Park. Similarly, Steller lions aggregate and forage on spring-spawning herring in Sitka Sound (Figure 4) near Sitka National Historical Park (Womble et al. 2005).

NPS/Jamie Womble
California sea lions (Zalophus californianus), a pinniped that is not particularly common in Alaska (Maniscalco et al. 2004), also seasonally migrate to the Alsek River in spring when eulachon are present. For example, the carcass of a male California sea lion that was marked on the Columbia River in Astoria, Oregon, and had previously traveled to San Miguel Island in California (Bryan Wright, Oregon Department of Fish and Wildlife, personal communication, June 2016), was documented south of the Alsek River in 2016.
Collectively, these examples demonstrate the influence of aggregations of spring-spawning forage fish on the distribution and migrations of pinnipeds in and adjacent to Alaska’s national parklands. The importance of the connections between spawning forage fish and far-ranging marine vertebrates, such as pinnipeds, demonstrates the importance of ecological connections in marine food webs regardless of management boundaries. Ultimately, variability in the scale and extent of aggregations of spawning forage fish may have significant implications for direct and indirect energy transfer throughout Alaskan marine ecosystems.
Acknowledgements
All research was conducted under NOAA Fisheries MMPA Permits # 16094-01, 782-1676-01, 14325, and 18537-01.
REFERENCES
Alerstam, T., A. Hedenström, and S. Åkesson. 2003.
Long-distance migration: evolution and determinants. Oikos 103:247-260. doi:10.1034/j.1600-0706.2003.12559.x
Bowen, W. D., O. T. Oftedal, and D. J. Boness. 1985.
Birth to weaning in four days: remarkable growth in the hooded seal, Cystophora cristata. Canadian Journal of Zoology 63(12):2841-2846.
Boyd, I. L. 1998.
Time and energy constraints in pinniped lactation. The American Naturalist 152: 717-728.
Costa, D. P. 1991.
Reproductive and foraging energetics of pinnipeds: implications for life history patterns. Pages 300-344 In D. Renouf, ed. The behaviour of pinnipeds. Springer Netherlands.
Jönsson, K. I. 1997.
Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78: 57-66.
LeBoeuf, B. J. , D. E. Crocker, D. P. Costa, S. B. Blackwell, P. M. Webb, D.H. Hauser 2000.
Foraging ecology of northern elephant seals. Ecological Monographs 70: 353-382.
Maniscalco, J. M., K. Wynne, K. W. Pitcher, M. B. Hanson, S. R. Melin, and S. Atkinson. 2004.
The occurrence of California sea lions (Zalophus californianus) in Alaska. Aquatic Mammals 30: 427-433.
Marra, P. P., E. B. Cohen, S. R. Loss, J. E. Rutter, and C. M. Tonra. 2015.
A call for full annual cycle research in animal ecology. Biology Letters 11: 20150552.
Mathews, E. A. and G. W. Pendleton. 2006.
Declines in harbor seal (Phoca vitulina) numbers in Glacier Bay National Park, Alaska, 1992-2002. Marine Mammal Science 22:170-191.
Mathews, E. A., J. N. Womble, G. W. Pendleton, L. A. Jemison, J. M. Maniscalco, and G. Streveler. 2011.
Population expansion and colonization of Steller sea lions in the Glacier Bay region of southeastern Alaska: 1970s to 2009. Marine Mammal Science 27: 852-880.
Milner-Gulland, E. J., J. M. Fryxell, and A. R. Sinclair, eds. 2011.
Animal migration: A synthesis. Oxford University Press.
Oftedal, O. T., D. J. Boness, and R. A. Tedman. 1987.
The behavior, physiology, and anatomy of lactation in the pinnipedia. Pages 175-245 In Genoway, H. M, ed. Current Mammalogy. Plenum Publishing Corporation.
Rehberg, M. J., L. A. Jemison, J. N. Womble, and G. O’Corry-Crowe. (In revision)
Winter movements and long-term dispersal of Steller sea lions in the Glacier Bay region of southeastern Alaska. Endangered Species Research.
Robinson, P. W., D. P. Costa, D. E. Crocker, J. P. Gallo-Reynoso, C. D. Champagne, M. A. Fowler, C. Goetsch, K. T. Goetz, J. L. Hassrick, L. A. Hückstädt, and C. E. Kuhn. 2012.
Foraging behavior and success of a mesopelagic predator in the northeast Pacific Ocean: insights from a data-rich species, the northern elephant seal. PLoS ONE 7(5), p.e36728.
Sigler, M. F., S. M. Gende, and D. J. Csepp. 2017.
Association of foraging Steller sea lions with persistent prey hot spots in southeast Alaska. Marine Ecology Progress Series 571:233-243.
Sigler, M. F., J. N. Womble, and J. J. Vollenweider. 2004.
Availability to Steller sea lions of an ephemeral prey resource, a pre-spawning aggregation of eulachon in southeastern Alaska. Canadian Journal of Fisheries and Aquatic Sciences 61: 1475-1484.
Surma S., E.A. Pakhomov, and T. J. Pitcher. 2018.
Energy-based ecosystem modelling illuminates the ecological role of Northeast Pacific herring. Marine Ecology Progress Series 588:147-161.
Willson, M. F. and J. N. Womble. 2006.
Vertebrate exploitation of pulsed marine prey: a review and the example of spawning herring. Reviews in Fish Biology and Fisheries 16:183-200.
Womble, J. N. and S. M. Gende. 2013.
Post-breeding season migrations of a top predator, the harbor seal, from a marine protected area in Alaska. PLoS ONE 8(2): e55386. doi:10.1371/journal.pone.0055386
Womble, J. N., G. W. Pendleton, E. A. Mathews, G. M. Blundell, N. M. Bool, and S. M. Gende. 2010.
Harbor seal decline continues in the rapidly changing landscape of Glacier Bay National Park, Alaska, 1992-2008. Marine Mammal Science 26:686-697.
Womble, J. N., M. F. Sigler, and M. F. Willson. 2008.
Linking seasonal distribution patterns with prey availability in a central-place forager, the Steller sea lion. Journal of Biogeography 36: 439-451.
Womble, J. N., M. F. Willson, M. F. Sigler, B. P. Kelly, and G. R. Van Blaricom. 2005.
Distribution of Steller sea lions in relation to spring-spawning fish species in SE Alaska. Marine Ecology Progress Series 294: 271-282.
Yang, L. H., K. F. Edwards, J. E. Byrnes, J. L. Bastow, A. N. Wright, and K. O. Spence. 2010.
A meta-analysis of resource pulse-consumer interactions. Ecological Monographs 80: 125-151.
Last updated: June 12, 2018
