Last updated: December 4, 2023
Article
Manhattan Project Science at Hanford

US DEPARTMENT OF ENERGY
One of those methods was based on using enriched uranium to fuel the bomb. The uranium enrichment was done at the Clinton Engineer Works in Oak Ridge, Tennessee. The second method involved producing large quantities of the recently discovered element plutonium. Unlike uranium, plutonium was almost nonexistent in nature, but it could be created in nuclear reactors. And that job would be done at the Hanford Engineer Works in southeastern Washington state.
The manufacturing process at Hanford was developed from what Enrico Fermi and his team proved when they constructed the world’s first, albeit small-scale, nuclear reactor in Chicago in 1942. If a reactor could be built sufficiently large, the intense flow of neutrons within it could, almost magically, change uranium into plutonium. This process of transmutation would not be creating gold from straw or lead but would be creating something much more valuable.
US DEPARTMENT OF ENERGY
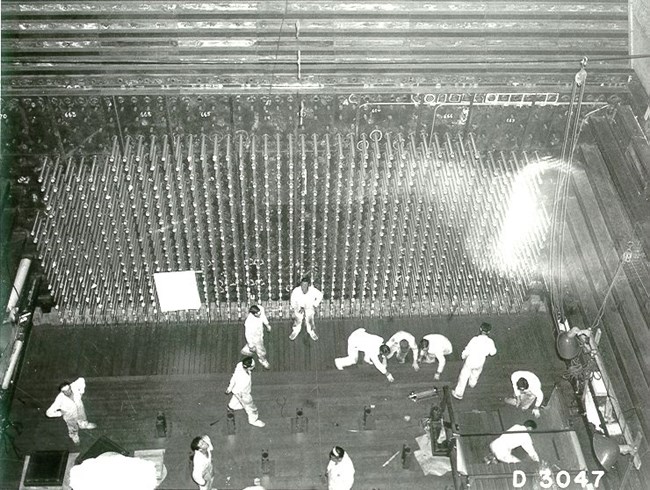
First, tons of uranium would be formed into 8.7-inch rods (22 cm), about 1.5 inches (3.81 cm) in diameter. Each was then clad in aluminum in a process known as canning. The result was commonly referred to as a fuel slug, tens of thousands of which would be made for the next step of the process.
The next step was to irradiate the fuel slugs in a nuclear reactor. The B Reactor was the first of three plutonium production nuclear reactors built at Hanford during World War II. At the core of each reactor was a huge matrix of graphite blocks, measuring 36 feet (10.97 m) by 36 feet (10.97 m) by 28 feet (8.53 m) front to back, and enclosed in 5 feet (1.52 m) of heavy shielding. From front to back ran 2004, 1.7-inch (51.81 cm) aluminum process tubes, into which were loaded more than 60,000 uranium fuel slugs. Cooling water from the Columbia River would flow in the narrow space between the fuel slugs and the process tubes. Enrico Fermi supervised the first loading of uranium slugs into the B Reactor on September 18, 1944. The B Reactor achieved criticality on September 26, 1944. A reactor achieves criticality (and is said to be critical) when each fission event releases a sufficient number of neutrons to sustain an ongoing series of reactions.
The graphite blocks in the core of a nuclear reactor slow down the fast neutrons released during the fission of uranium 235 (U-235). The fission process generates neutrons that initiate fission in other U-235 atoms in a continual process of splitting U-235 atoms and releasing more neutrons. Soon, there are enough slowed neutrons to create a controlled sustained nuclear chain reaction. Some of the free neutrons are absorbed by uranium 238 (U-238) atoms becoming uranium 239 (U-239). The U-239 atoms then transmute (change into) into neptunium 239 (Np-239). Np-239 transmutes into plutonium 239 (Pu-239), the product of interest.

US DEPARTMENT OF ENERGY
Every four to six weeks of operation, workers pushed about 10-20 percent of the now highly radioactive fuel slugs out of the back of the reactor and into the water-filled fuel storage basin where they would thermally and radiologically cool off for approximately two to three months. After the cooling off period, the still highly radioactive fuel slugs were loaded into shielded, water-filled casks on train cars. They were then transported to the T Plant where multiple chemical processes would separate the plutonium from the uranium and other radioactive byproducts produced during irradiation. Dissolving the aluminum jacket around the fuel slugs and separating plutonium from the uranium and other radionuclides produced during irradiation required more than a dozen steps in the chemical separations process. Approximately 4000 pounds (1814.36 kg) of uranium were needed to produce 1 pound (0.45 kg) of plutonium. That is like reducing the weight of an elephant at 12,000 pounds (5443.10 km) down to the weight of a kitten at 3 pounds (1.36 kg).
Once the plutonium was extracted, the chemically separated uranium, unwanted radionuclides, and chemicals used in the process became liquid waste and were put into underground waste storage tanks at Hanford. The work during World War II focused on refining the process for chemically separating plutonium from uranium for the war effort. Addressing the chemical waste was put off until after the war. The mix of metals, chemicals, and radioactivity in the nuclear and chemical waste at Hanford lead to a serious and very expensive clean-up process still being dealt with today—more than seven decades later.
The plutonium was carefully and secretly shipped to the Manhattan Project site at Los Alamos, New Mexico in multiple shipments. There, scientists, engineers, and craft workers designed and built a device, known as the Gadget, to test an implosion-design plutonium-fueled atomic bomb. The Gadget used Hanford’s plutonium and was successfully detonated during Trinity test in New Mexico on July 16, 1945. The Trinity test was the first human-caused nuclear explosion in history and ushered in the nuclear age. A few weeks later on August 6, the Little Boy atomic bomb, fueled by enriched uranium from Oak Ridge, was detonated over Hiroshima, Japan, the first atomic weapon used in war. And then on August 9, 1945 the US dropped the Hanford plutonium-fueled Fat Man bomb over Nagasaki, Japan. This was the second atomic bomb used on a human population and, so far, the last.
- Rhodes, Richard. The Making of the Atomic Bomb. New York: Simon & Schuster, 1986.
- Smyth, Henry De Wolf. Atomic Energy for Military Purposes, 1945. California: Stanford University Press, 1989
