Part of a series of articles titled Alaska Park Science - Volume 20, Issue 2. Beringia: A Shared Heritage.
Article
The Aleutians: Observing Recent Floristic Changes Along the Stepping Stones of the North Pacific

Photo courtesy of Eric DeChaine
The islands of the Aleutian Archipelago emerge like stepping stones across the North Pacific, linking Asia and North America (Hutchinson 1937; Figure 1). The associated peninsulas and islands of these North Pacific Stepping Stones (NPSS), including Kamchatka in the far west, the Commander (Komandorsky)-Aleutian Islands, and the Alaska Peninsula to the east, have provided a migration route for terrestrial taxa between the continents since the Tertiary Period. The Kamchatka Peninsula juts out roughly 775 miles (1,250 km) from the Russian mainland southward into the western Pacific Ocean, dividing the Okhotsk and Bering seas. The Commander Islands, located in the northwestern Pacific Ocean, 100 miles (175 km) east of the Kamchatka Peninsula, are somewhat isolated at the western extreme of the Aleutian Islands. The approximately 300 islands of the Aleutian Archipelago arc across the North Pacific between 51° and 56° N latitude. The mountainous Alaska Peninsula extends southwest roughly 500 miles (800 km) from the Alaska mainland. Kodiak Island, the 2nd largest island in the U.S., sits about 25 miles (40 km) to the south of the peninsula.
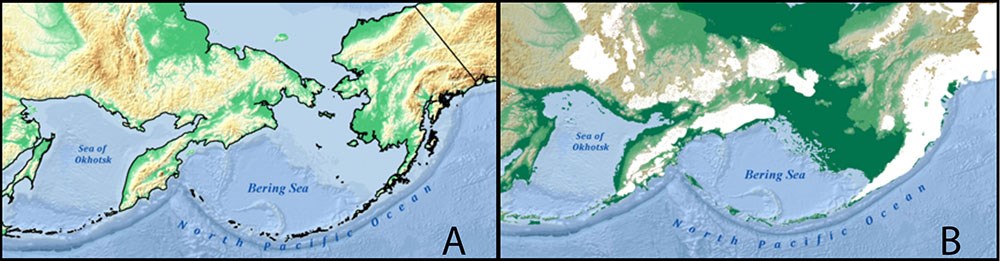
A. Current sea level (blue) with degree of shading indicating bathymetry. B. Glacial extent (white) during the last glacial maximum (LGM, about 18,000 years ago) when sea level was approximately 400 feet (120 m) below current levels (green and blue shade)
The NPSS would have persisted above sea level throughout both warm and cold periods of the Pleistocene epoch, though never as a continuous bridge (Figure 1). During warm periods, sea levels rose, islands shrank, and the archipelago became more fragmented with greater distances between landmasses. But those inter-glacial periods provided more suitable environmental conditions for most species. Alternatively, with the lowered sea levels of glacial periods, neighboring islands would have been connected into larger, though mostly glaciated, landmasses (Hamilton 1994), separated only from the mainland and other island groups by deep waterways (e.g., Hultén’s line, Commander Gap, Buldir Gap, Amchitka Pass, Amukta Pass; Lindroth 1961, Hultén 1937, Garroutte 2016, Heusser 1990, Tatewaki and Kobayashi 1934). At such times, the central Aleutians (Rat and Andreanof Islands) may have been a refugium (Frenzel et al. 1992, Pruett and Winker 2007), an ecologically stable area that could have provided suitable habitat throughout the glacial cycles. The eastern Aleutians were connected to the heavily glaciated Alaska Peninsula, though Kodiak Island was unglaciated on its northwestern side and harbored a glacial refugium (Karlstrom and Ball 1969). In the west, Kamchatka was partially glaciated, but still distant from the Commander Islands (Frenzel et al. 1992). Thus, the stepping stones would have persisted across the North Pacific throughout the glacial cycles, but migration and the potential for refugia along the NPSS route would have varied depending on oscillations in island size, distance between landmasses, and the extent of glaciers.
Indeed, the Aleutian Islands (what we refer to as the North Pacific Stepping Stones) have been a critical route across the North Pacific for the migration and diversification of flora and fauna, including humans, since the last glacial maximum (LGM). Human movement through the Aleutian Archipelago may have facilitated the earliest expansion of human populations into North America, especially along a sea route (Erlanson et al. 2007, Wade 2017). En route, local island floras provided food and material for the early colonists (Veltre et al. 2006). And with humans came the potential movement of additional important edible and medicinal plants. Occurrences of such species on certain islands may be closely tied with Indigenous settlement of those islands or proximity of village sites where frequent collection and gathering enhanced plant populations (Bank 1953).
Flora of the North Pacific Stepping Stones
Today, the NPSS hosts a subarctic maritime tundra flora that overlaps with, but differs from, that in the more continental Beringia region due to a long history of a warmer, wet, and windy climate. The paleogeographic history of the NPSS has promoted a relatively diverse flora, for the latitude, with defined geographic distribution. Descriptions of the Kamchatka (Hultén 1927), Commander (Hultén 1968), Aleutian (Hultén 1960, Tatewaki and Kobayashi 1934), Alaskan (Hultén 1968), and Kodiak (Karlstrom and Ball 1969) floras illustrated the basic plant distributions for identifying patterns across the region.
Hultén (1927) described the Kamchatka Peninsula as an “island” of temperate vegetation, ranging from coastal woodlands to tundra, with about 1,100 species of vascular plants including over 40 that are now considered rare or endangered, and several endemics. The treeless Commander Islands host 432 species and subspecies of vascular plants in an area of disputable phytogeographic affinities (Hultén 1960, Volkova et al. 2016). Likewise, the Aleutian Islands are treeless (with isolated introductions of Sitka spruce), but more diverse, including about 520 species, 37 of which are endemic (Lindroth 1961, Golodoff 2003, Garroutte 2016, Tatewaki and Kobayashi 1934). Approximately 685 species inhabit the diverse landscape of the Alaska Peninsula, which is one of the richest communities in Beringia (Hultén 1968, Ickert-Bond et al. 2013). On the nearby Kodiak Island refugium, over 450 species of vascular plants can be found (Karlstrom and Ball 1969).
And yet, our understanding of Aleutian flora remains limited. Present day flora is inadequately documented and thus the past and potential future of plant distributions across the bioregion remain uncertain. The remoteness and difficult conditions across the Aleutian chain and adjacent continental areas have reduced the level of scientific inquiry along this route. Botanical surveys have been focused on specific areas, with opportunistic sampling due to logistical issues (Yakubov 2007). We know little about historic and contemporary species distributions across the archipelago, where they occur on each island, and whether they are abundant or rare, cosmopolitan or endemic.

Photo courtesy of Eric DeChaine
Recent Human-induced Disturbances
Ever since the description and mapping of the island chain by the European explorer, Captain Vitus Bering, in the 18th century, the Native peoples living there have experienced substantial change to the native animals and plants which share their homeland (Bank 1953, West 2012). Other examples of disturbances brought along with European hunters to the Aleutian ecosystems exist. Europeans overhunted Steller’s Sea Cow (Hydrodamalis gigas) into extinction by 1741 (Murie 1959). Russian conscription of the Unangan (Aleut) people during the sea otter and fur seal harvests and later fox “farming” decimated many of the Unangan communities (Hutchinson 1937, Collins et al. 1945) and led to disruption of their land use and ethnobotanical needs. The sea otter (Enhydra lutris) populations collapsed from over-hunting. Russians introduced blue fox (Alopex lagopus) from the mainland for fur production to islands previously lacking foxes, which devastated seabird colonies (Murie 1959). The fur trade, as well as naval traffic, also brought Norway rats which have decimated bird and plant populations on previously rat-free islands (Murie 1959).
A number of the larger central and western islands in the Aleutians were ravaged during World War II, beginning with the invasion of Attu, Kiska, and Agattu by the Japanese Imperial forces in 1942-43, and the subsequent U.S. response through the remaining islands leading up to the western points of conflicts (Morgan 1980). The Attu villagers were taken to Japan as prisoners of war (Golodoff 2012, Morgan 1980). Those few that survived the war years returned to the Alaskan mainland and later to Unalaska, Atka, and other eastern villages. Attu has not been resettled (Morgan 1980).
The Unangax̂ to the east were moved to avoid any exposure to military actions during the Japanese occupation and subsequent U.S. military responses, this time by the U.S. Army, 1,500 miles (2,400 km) from their homes to mainland Alaska. Relocations after the war were attempted by the government and Native communities, but previous village populations were permanently disrupted in many cases (Morgan 1980, Golodoff 2012). Nuclear tests were performed on Amchitka Island for the U.S. Atomic Energy Commission (AEC) in the late 1960s through early 1970s with potentially catastrophic environmental impacts (Morgan 1980).
The township of Adak, a previous U.S. Navy base, closed in 1994 and ownership of the northern island and built facilities were transferred to the Aleut Corporation. Clean-up work continues on the base closure at Adak as well as at numerous past military sites throughout the Aleutian chain (USFWS, Lisa Spitler, personal communication). Many of these clean-up projects involve removal of stored hazardous waste and other contaminants, with follow-up revegetation work as needed. Pre-1970s restoration or land stabilization efforts associated with the military base on Adak did not require native species to be used in plantings and inadvertent naturalization occurred among the non-native species used, such as white clover and numerous fescue and blue grasses. In 1977, an executive order (Carter 1977) required use of native species in restoration plantings on all federal lands. Since this time, it is apparent that native species as well as some introduced species have been used as needed for restoration associated with on-going clean-up activities (observations from Williams in the 2019 field season). These are but a handful of examples of factors that have confounded our understanding of the original pre-disturbance flora.
Comparing the Flora of Adak: The Late 1970s to 2019
We targeted the island of Adak for a comparative botanical investigation, given Williams’s work there in the late 1970s (Williams 1980). The collections and identifications from 1977-78 (Williams et al. in prep.) and 2019 bring the vascular flora of Adak Island to around 220 native taxa (Williams et al. in prep), almost twice what was previously thought (Garroutte et al. 2018). This includes 49 families (219 taxa) of vascular plants (Williams et al. in prep). Approximately 20 plants are new records to Adak, 10 or so new to central Aleutians, and a few even new to central and northern Alaska (Table 1).
| New to Adak (post Hultén 1968, 1973) |
Collector and Year Collected |
|---|---|
| Polystichum aleuticum | D. K. Smith, U. Tenn, 1975 |
| Polystichum lonchitis | M. P. Williams 1978, 2019 |
| Adiantum aleuticum | M. P. Williams 1978 |
| Carex gynocrates | M. P. Williams 1978, 2019 |
| Carex sitchensis | M. P. Williams 2019 |
| Juncus castaneus ssp. leucochlamys | Clebsch 1978 |
| Luzula arcuata ssp. unalaschensis | M. P. Williams 2019 |
| Elymus dahuricus (?) | M. P. Williams 1978, 2019 |
| Torreyochloa pallida var. pauciflora | Clebsch 1978 |
| Cypripedium guttatum | M. P. Williams 1978 |
| Spiranthes romanzoffiana | M. P. Williams 1978 |
| Artemisia tilesii | M. P. Williams 1978 |
| Draba fladnizensis | Clebsch 1978 |
| Moehringia lateriflora | M. P. Williams 1978 |
| Montia fontana | M. P. Williams 2019 |
| Stellaria crassifolia | M. P. Williams 2019 |
| Epilobium leptocarpum | M. P. Williams 2019 |
| Hippuris montana | M. P. Williams 1978 |
| Koenigia islandica | M. P. Williams 1978 |
| Ranunculus eschscholtzii | M. P. Williams 1978 |
| Sanguisorba stipulata | M. P. Williams 1978 |
Not all of our findings are promising however, because of the introduced non-native species. On Adak, Williams collected what might be established populations of a non-native perennial grass, Elymus dahuricus, of Asian origin and not previously known from the Aleutian Chain (Hultén 1968, 1973). It was found growing with the native American dunegrass, Leymus mollis, at a number of inland remote sites away from any recent restoration plantings. This species has been listed for reclamation plantings in the U.S. (plants.usda.gov as Dahurian Wildrye) and Canada (Dobb and Burton 2013). Further analyses are needed to confirm if any such hybrids with the native Leymus mollis are present or if this species or any other hybrid-forming grasses have been used in the past for revegetation in the Aleutian Islands. We hope to verify the genetic and relational status of these populations with those of the native dune grass in the near future.
Observations of the Potential Consequences of Environmental Change
We were able to document some of the environ-mental changes that may have impacted the flora by comparing our 2019 survey data with that from the late 1970s (Table 2, Figure 2). The observed changes are likely a result of several factors, including the ongoing impacts of non-native taxa and global warming.
| Change in Organisms | 1977-78 | 2019 |
|---|---|---|
| Lichen-Empetrum mats | Approximately 30 cm thick in upland swales between ridges | 2-3 cm thick |
| Caribou population (from annual caribou census, USFWS staff, personal communication to M. P. Williams; West 2012, Liebermann et al. 2015) | 250-275 | 1,500-2,500 with some colonization of neighboring Kagalaska Island |
| Cryptobiotic crusts | Prevalent | Absent or Rare |
| Hoof action on solifluction terraces | Low | High |
| Rat population | Moderate | High |
| Occurrence of introduced foxes from fur trade | High | Believed to be exterminated by USFWS eradication program up to 2018 (from USFWS, Lisa Spitler, personal communication to Williams) |

A. Caribou carcasses following winter die-off [or population collapse] on ridge between Finger and Thumb bays, July 2019.
Photo courtesy of Cullen Williams
B. Abundant gas bubbles coating the submerged surface of shallow ponds were frequently observed in the 2019 field season. This was not obvious or commonly seen during the 1977-78 field seasons.
Photo courtesy of Mike Williams
C and D. Change in cover of cryptobiotic crusts on the southern slopes of Mount Moffett. Note (C) presence and (D) absence of cryptobiotic crust on the soil surface. Both photographs were taken in July at 365 meters ASL on south-facing slopes with extensive solifluction terraces. In addition, Salix arctica mats that commonly extended out on these terraces in 1977-1978 were mostly absent or restricted to the margins of the terraces in 2019.
C photo from 1977 courtesy of Mike Williams
D 2019 photo courtesy of Matt Richards-Perhatch
E and F. Shoreline erosion of Kuluk Bay foredune and first terrace/dune swale.
(E) In this image from 1977, the wide expanse of the first terrace is reduced in 2019 to half the width with no discernable foredune on the beach edge (red arrow denotes approximate edge of erosional face in 2019).
Photo courtesy of Mike Williams
(F) Dune face towards Kuluk Bay showing erosional loss of foredune and dune terrace/swale.
Photo courtesy of Mike Williams
G and H. Changes in abundance of Anemone narcissiflora on the southern slopes of Mount Moffett in early July (G) 1977-78 to (H) 2019
Photos courtesy of Mike Williams
Introduced barren-ground caribou (Rangifer tarandus granti) and Norway rat (Rattus norvegicus) populations have risen dramatically on Adak since the naval base closure (Table 2). The caribou were introduced to Adak as a game animal in the 1950s and have persisted to the present (Liebermann et al. 2015, Ricca et al. 2014). Simultaneously, globally, summer temperatures have increased (Voosen 2021). Reindeer lichens, cryptobiotic crusts on solifluction terraces (Figure 2), and some vascular plants (Figure 2) have decreased in cover and abundance (Table 2). On Saint Matthew Island, approximate 8° latitude north of Adak Island, it was noted that, in areas of heavy winter utilization by introduced reindeer:
Lichen growth, which formerly occupied the slight depressions between raise hummocks of prostrate willows, had been almost completely removed (Klein 1959).
Mack and Thompson (1982) stated that:
... in communities… in which cryptogram cover is extensive, these slow growing organisms [lichens, algae, and mosses of cryptobiotic crusts] respond adversely to regular grazing by large animals.
They further stated that :
Maintenance of cryptograms in boreal and arctic regions is apparently dependent on comparably low continuous ungulate activity by migratory herds…(Mack and Thompson 1982).
The caribou population on Adak in the last 20 years fluctuated around the highest population observed (West 2012). Individuals even were migrating for the first time to neighboring Kagalaska Island (Liebermann et al. 2015). This strongly suggests that the caribou herd on Adak, introduced in the 1950s, has likely exceeded an ecological carrying capacity for this island as has previously been observed by others (Liebermann et al. 2015, Ricca et al. 2014).
Trampling from caribou and other ungulates at high population density coupled with global warming are both known to detrimentally impact the maintenance and continued growth or persistence of cryptobiotic crusts (Turunen et al. 2009, Concostrina-Zubiri et al. 2014, Olofsson 2006, Ricca et al. 2016). A review of the July mean temperature collected at the Adak airport for ten-year intervals over the last 70 years suggested an increase from 48°F to 51°F (9.2°C to 10.4°C). In 1978, Williams observed rapid heating on the dark, organic-rich exposed soils of the terraces in excess of 95°F (35°C) within 30 minutes of incubation in nitrogen fixation chambers, with ambient temperatures of 41-45°F (5-7°C; M. Williams, unpubl. data). The denuded dark solifluction terraces likely act to absorb abundant infrared (IR) radiation in the mostly fog-covered landscape. The loss of cryptobiotic crusts, which otherwise hold the open soil surface in place, likely has resulted in increased erosion of the solifluction terraces.
During the 2019 field season we observed abundant “pocketing” or torn openings in the otherwise dense tundra mats throughout central and northern Adak. These pockets, often associated near rat skulls or other skeleton parts, were not observed in any abundance in 1977-78 by Williams. Species of angiosperms showing declines in abundance from 1977-78 to 2019 included Anemone narcissiflora, Dactylorhiza aristata, Platanthera convallariaefolia, Spiranthes romanzoffiana, Fritilleria camschatcensis, Streptopus amplexifolius, and Caltha palustris (Williams et al. in prep). Most of these species are edible, or some are at least medicinal, for humans (Veltre et al. 2006). Again, the increased caribou herd size and abundance of introduced rats may explain these plant declines. Murie (1959) specifically noted that on Atka Island:
…we found large areas where the rats had eaten the basal parts of the stems of Anemone narcissiflora.
Gas bubbles observed in ponds of the upland slopes in 2019 may be associated with increased soil decomposition tied to global warming (Figure 2B). Further study is needed to determine if the concentration and abundance of these bubbles may be due to an increased release of organic gases such as methane from increased decomposition of organic-rich soils on Adak and possibly elsewhere in the Aleutian Islands. This pattern was not observed in 1977-78 by Williams. Any loss of stored soil organic matter through increased decomposition from climate warming will likely result in changes to the vegetation structure and diversity on Adak in the future.
By 2019, Williams observed that the erosional face of Kuluk Beach, a sand dune system northeast of the Adak townsite, has steepened and progressed inland, removing most of the foredune and first terrace surveyed in 1977-78 (Williams 1980). This change has resulted in a loss of 33-45 feet (10-14 m) width of the front edge of the vegetated dunes. Also, there is no longer an interdune swale, which in the past had seasonally exhibited a shallow linear freshwater pond on this first terrace (Williams 1980; Figure 2 E and 2F). Such shoreline retreat may signal increased erosion to unique native habitats with climate change.
Further Study is Needed
Our recent observations and collections suggest that we do not have a complete picture of the Aleutian flora and how it is being impacted by environmental changes following European contact. The abundance of many dominant plants and characteristics of vegetation in parts of the landscape observed by Williams in the late 1970s is different than what we saw in 2019. Botanical inventories are needed in high latitudes where the impact of global climate change is especially acute and where development is accelerating in the United States and Russia. Warming is opening up the Arctic to further human use. Shipping traffic in the North Pacific and through the Aleutian Islands is projected to increase dramatically, with an increased probability of intro-duced species. Invasive species already occur in the islands. Profound warming (IPCC 2014, Voosen 2021) is influencing the geographic distribution of the northern biotic communities. As such, there is a critical need to document the current distribution of species and explore the processes of diversification, colonization, and extinction of native species of plants, as well as animals, around the North Pacific. Also, it is important to recognize that the Unangax̂ people are resilient and have worked to preserve their technologies of native medical, edible, and other useful plants for future generations (Golodoff 2003, Unger 2014).
Globally, island biota face the highest rates of extinction, where 80% of all recorded extinctions are from islands (Ricketts et al. 2005). Island populations tend to be small, geographically restricted, vulnerable to establishment of invasive species, subjected to higher rates of inbreeding, and lack effective dispersal corridors to track suitable ecological niche space through time. These conditions lead to high vulnerabilities of island biota, particularly when coupled with habitat conversion, harvest, or other anthropogenic activities. Importantly, 25% of the globally rare to imperiled plant species in Alaska are restricted to the Aleutian and Bering Sea islands (AKNHP 2018) despite the small area. Our findings underscore the need for more thorough surveys, hint at potential novel discoveries, and suggest that more dramatic change is on the way. Informed conclusions regarding human impacts on natural populations and the effects of climate change at high latitudes will require a detailed understanding of the distribution and status of the flora. We cannot manage for future changes without a current understanding of the flora from which to gauge the response of plants to environmental shifts.
Acknowledgements
We thank the Aleut Corporation and U.S. Fish and Wildlife Service, Alaska Maritime Refuge, in particular, Jeff Williams and Lisa Spitler, for access to Adak Island and for housing and use of a vehicle during our field studies. In addition, we thank the Alaska Volcano Observatory for assistance traveling to and from Tanaga Island. Without them that aspect of our field research would not have been possible. We also thank the Shared Beringia Heritage Program and the National Park Service for providing the necessary funding for this research. Special thanks to the three student volunteers, Matt Richards-Perhatch, Thomas Barlow, and Cullen Williams, for their field assistance on Adak Island.
References
Alaska Natural Heritage Program (AKNHP). 2018.
Alaska Rare Plant Data Portal. Alaska Natural Heritage Program, Alaska Center for Conservation Science, University of Alaska Anchorage. Available at: http://aknhp.uaa.alaska.edu/apps/rareplants/ (accessed 20 January 2018)
Bank, T. P., II. 1953.
Ecology of prehistoric Aleutian village sites. Ecology 34: 246-264.
Carter, J. 1977.
Presidential documents: Executive Order 11987—Exotic Organisms. Federal Register 42: 26949-26950.
Collins, H. B., A. H. Clark, and E. H. Walker. 1945.
The Aleutian Islands: Their people and natural history. Smithsonian Institution Publication 3775, Washington, D.C.
Concostrina-Zubiri, L., E. Huber-Sannwald, I. Martinez, J. L. Flores Flores, J. A. Reyes-Aguero, A. Escudero, and J. Belnap. 2014.
Biological soil crusts across disturbance-recovery scenarios: Effect of grazing on community dynamics. Ecological Applications 24: 1863-1877.
Dobb, A. and S. Burton. 2013.
Rangeland seeding manual for British Columbia. BC Ministry of Agriculture, Sustainable Agricultural Management Branch, Abbotsford, BC.
Erlanson, J. M., M. H. Graham, B. J. Bourque, D. Corbett, J. A. Estes, and R. S. Stenbeck. 2007.
The kelp highway hypothesis: marine ecology, the coastal migration theory, and the peopling of the Americas. Journal of Island and Coastal Archeology 2: 161-174.
Frenzel, B., M. Pécsi, A. A. Velichko, and L. Starkel. 1992.
Atlas of Palaeoclimates and Palaeoenvironments of the Northern Hemisphere. HAS, Budapest.
Garroutte, M. D. 2016.
Species Richness, Community Composition, and Species Distribution Patterns in Aleutian Plants. Thesis, University of Alaska Fairbanks.
Garroutte, M., F. Huettmann, C. O. Webb, and S. M. Ickert-Bond. 2018.
Biogeographic and anthopogenic correlates of Aleutian Islands plant diversity: A machine-learning approach. Journal of Systematics and Evolution 56: 476-497.
Golodoff, N. 2012.
Attu Boy. U.S. Department of the Interior, National Park Service, Aleutian World War II National Historic Landmark, special publication.
Golodoff, S. 2003.
Wildflowers of Unalaska Island: A Guide to the Flowering Plants of an Aleutian Island. University of Alaska Press, Fairbanks, AK.
Hamilton, T. D. 1994.
Late Cenozoic glaciation of Alaska. The Geology of Alaska (ed. G. Plafker, H. Berg), pp. 813–844. The Geological Society of America, Boulder.
Heusser, C. J. 1990.
Late Quaternary vegetation of the Aleutian Islands, southwestern Alaska. Botany 68: 1320-1326.
Hultén, E. 1927.
Flora of Kamchatka and Adjacent Islands. Almqvist & Wiksell. Stockholm.
Hultén, E. 1937.
Outline of the History of Arctic and Boreal Biota During the Quarternary Period. Lehre J. Cramer, NY.
Hultén, E. 1960.
Flora of the Aleutian Islands and Westernmost Alaska Peninsula; With Notes on the Flora of the Commander Islands, 2nd Ed. J. Cramer, NY.
Hultén, E. 1968.
Flora of Alaska and Neighboring Territories, A Manual of the Vascular Plants. Stanford Univ. Press, Stanford, CA.
Hultén, E. 1973.
Supplement to Flora of Alaska and Neighboring Territories. Botaniska Notiser 126: 459-512.
Hutchinson, I. W. 1937.
Stepping stones from Alaska to Asia. Blackie & Son, London.
Ickert-Bond, S. M., F. Huettmann, I. Loera, L. Strecker, N. Sekretareva, and Y. Mikhailova. 2013.
New insights on Beringian plant distribution patterns. Alaska Park Science 12: 60-69.
Intergovernmental Panel on Climate Change (IPCC). 2014.
Climate Change 2014: Synthesis Report. Core Writing Team, R. K. Pachauri, L. A. Meyer (eds.). IPCC, Geneva, Switzerland.
Karlstrom, T. N. V. and G. E. Ball. 1969.
The Kodiak Island Refugium: Its Geology, Flora, Fauna, and History. The Ryerson Press, Toronto.
Klein, D. R. 1959.
Saint Mathew Island Reindeer-range study. U.S. Fish and Wildlife Service, Special Scientific Report-Wildlife No. 43. 48 p.
Liebermann, L., B. Hahn, and A. Carlson. 2015.
Aleutian Islands Wilderness: Preventing the establishment of non-native caribou on Kagalaska Island. U.S. Fish and Wildlife Service, Case Study of Ecological Restoration in Wilderness. 19 p.
Lindroth, C. H. 1961.
The Aleutian Islands as a route for dispersal across the North Pacific. Pacific Basin Biogeography (eds. Gressit, J. L. et al.), pp. 121-182. Bishop Museum Press, HI.
Mack, R. N. and J. N. Thompson. 1982.
Evolution in steppe with few large, hooved mammals. American Naturalist 119: 757-773.
Morgan, L. (ed.). 1980.
The Aleutian Islands. Alaska Geographic 3:130-161.
Murie, O. J. 1959.
Fauna of the Aleutian Islands and Alaska Peninsula. North America Fauna Series, No. 61, U.S. Fish and Wildlife Service, Washington, D.C. 406 p.
Olofsson, J. 2006.
Short- and long-term effects of changes in reindeer grazing pressure on tundra heath vegetation. Journal of Ecology 94: 431-440.
Pruett, C. L. and K. Winker. 2007.
Evidence for cryptic northern refugia among high- and temperate-latitude species in Beringia. Climatic Change 86: 23-27.
Ricca, M. A., A. K. Miles, D. H. Van Vuren, and V. T. Eviner. 2016.
Impacts of introduced Rangifer on ecosystem processes of maritime tundra on subarctic islands. Ecosphere 7: 1-22.
Ricca, M. A., D. H. Van Vuren, F. W. Weckerly, J. C. Williams, and A. K. Miles. 2014.
Irruptive dynamics of introduced caribou on Adak Island, Alaska: An evaluation of Riney-Caughley model predictions. Ecosphere 5(8): 94.
Ricketts, T. H., E. Dinerstein, T. Boucher, et. al. 2005.
Pinpointing and preventing imminent extinctions. PNAS 102: 18497-18501.
Tatewaki, M. and Y. Kobayashi. 1934.
A contribution to the flora of the Aleutian Islands. J. Fac. Ag., Hokkaido Imperial Univ. 36: 1-119.
Turunen, M., P. Soppela, H. Kinnunen, M. L. Sutinen, and F. Martz. 2009.
Does climate change influence the availability and quality of reindeer forage plants? Polar Biology 32: 813-832.
Unger, S. 2014.
Qaqamiiĝux: Traditional Foods and Recipes from the Aleutian and Pribilof Islands. Aleutian Pribilofs Islands Association. 381 p.
Veltre, D. W., C. L. Pendleton, S. A. Schively, J. A. Hay, and N. Tararenkova. 2006.
Aleut/Unangax ethnobotany: An annotated biography. Conservation and Arctic Flora and Fauna (CAFF) Technical Report No. 14, CAFF International Secretariat, Akureyi, Iceland.
Volkova, P. A., A. A. Bobrov, Y. O. Kopylov-Guskov, et al. 2016.
Notes on the flora of the Commander Islands. J. Bot. 101: 829-842.
Voosen, P. 2021.
Global temperatures in 2020 tied to record highs: defying cooling from La Niña, warming planet speeds toward breach of climate targets. Science 371: 334-335.
Wade, L. 2017.
Most archeologists think the first Americans arrived by boat. Now they’re beginning to prove it. Science News, Available at: https://www.sciencemag.org/news/2017/08/most-archeologists-think-first-americans-arrived-by-boat-now-they-re-beginning-to-prove-it (accessed 01 March 2021)
West, D. 2012.
An introduction to Adak, Alaska, pp. 1-5. In D.West, V. Hatfield, E. Wilmerding, C. Lafèvre, and L. Gualtieri (eds.) The People Before: The Geology, Paleoecology, and Archeology of Adak Island, Alaska. Archaeopress, Oxford, England.
Williams, M. P. 1980.
Studies of Elymus mollis directed toward its use in revegetation of maritime tundra. Master thesis, University of Tennessee, Knoxville. 123 p.
Williams, M. P., C. C. Amundsen, E. C. C. Clebsch, and S. Schulmeister. In Prep.
Annotated checklist of the vascular plants of Adak Island, Alaska.
Yakubov, V. 2007.
Plants of Kamchatka (The Field Guide). Moskva: Put’ Istina i Zhizn.
Last updated: December 15, 2021
