|
NEW RIVER GORGE
PROCEEDINGS New River Symposium 1984 |

|
THE ECOLOGY OF BLACK FLIES IN THE NEW RIVER AND ITS MAJOR TRIBUTARIES IN SOUTHERN WEST VIRGINIA
J. Reese Voshell, Jr.
Department of Entomology
Virginia Polytechnic Institute and State University
Blacksburg, VA 24061
INTRODUCTION
Adult black flies are adept fliers that measure about 2-3 mm long. Females must feed on blood for their eggs to develop. Different species are known to feed on a variety of birds and mammals, sometimes including man. The females locate their preferred hosts by odors, visual stimuli, and carbon dioxide exhaled by the host. Even when the females do not feed on a particular host, they are sometimes attracted and swarm in annoying numbers around the host's head. The immature stages, called larvae, develop only in flowing water. They attach to firm substrates such as rocks or vegetation, by spinning silk on the substrate and then holding the silk with small hooks on the posterior of their bodies. The larvae feed on very fine particles of organic matter (0.01 to 0.1 mm) that they filter from the water as current passes through fan-like structures on their heads. After the larvae have finished growing, they pass through a resting stage (pupa) in a silk cocoon, and then they transform into adults which rise to the surface and fly away. For more detailed information on the biology of black flies, readers are referred to recent reviews by Colbo and Wotton (1981), Cupp (1981), Peterson (1981), and Wenk (1981).
During the past several years, considerable controversy has developed in southern West Virginia over black flies (Diptera: Simuliidae). Some citizens have complained vociferously about the "gnats" (adult black flies) that are present during the warm months of the year. Complaints include biting and general nuisance that prevent outdoor recreation such as picnics, golf, or tennis. Amrine (1982) reported that the problem was being caused by a single species, Simulium jenningsi, and he speculated that all of the pestiferous organisms were coming from a 7 mile section of the New River below Bluestone Dam. In response to complaints of some citizens, the State of West Virginia was asked to permit the application of bacterial pesticide, Bacillus thuringiensis israelensis (BTI), to the New River in order to kill the black fly larvae before they emerged from the water. However, other citizens of the area have expressed opposite opinions. Some persons do not consider black flies to be a serious nuisance, and they strongly object to the application of a pesticide into the New River. They fear that eliminating black flies from the river could have an adverse effect on the food web, and this might harm the very productive sport fishery found below Bluestone Dam.
In order to obtain the information necessary to respond to this controversy, the West Virginia Department of Natural Resources has contracted VPI&SU to conduct several studies on the ecology of black flies in southern West Virginia. If an attempt is made to manage the populations of black flies, it will be necessary to have a comprehensive ecological data base in order to choose the best method(s), to assure effective implementation of the method(s), and to monitor the success of the program. Conversely, it has been well documented in these symposia that the New River is a valuable natural resource, and there are commercial, recreational, aesthetic, and cultural reasons for its protection. The objectives of the research that we have been conducting are: (1) to analyze the species composition, distribution, and abundance of adult black flies; (2) to determine the breeding areas of the larvae; (3) to describe the life cycles of the important species; and (4) to determine the function of black flies in the New River ecosystem. The information that is presented in this paper is a summary of two reports on completed research (Kondratieff and Voshell 1933, Voshell 1983a) and a description of a study that is still in progress (Voshell 1983b).
METHODS
Adults
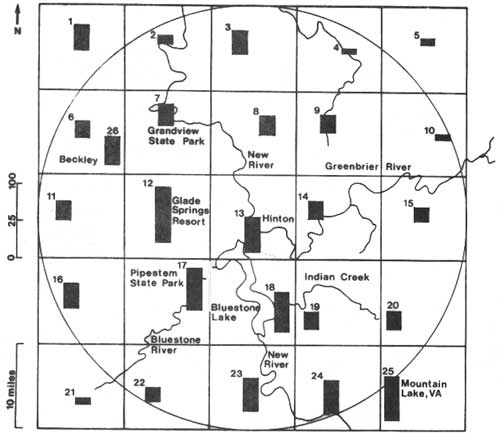
Our studies have been conducted throughout a 25 mile radius of Hinton. Adult black flies were sampled at 25 sites that were all approximate 10 miles apart (Fig. 1). These sites included several locations where black flies had been frequently reported to be a problem: Pipestem State Park, Grandview State Park, and Glade Springs Resort. A 26th site was established within the City of Beckley. Two quantitative methods were used to estimate populations of adult black flies. One method was to collect organisms in a large shaped net mounted on top of a van (Barnard 1979). This vehicle-mounted trap was driven at a constant speed over a known distance, thereby providing replicate quantitative samples (number of black flies per 1000m3 of air). The second method for collecting adult black flies was a trap with a fan that blew them down into a jar (Snoddy and Hays 1966, Fallis et al. 1967). Carbon dioxide was released from the trap to attract the black flies. The sampling method was made quantitative by releasing carbon dioxide at a constant rate and operating the trap for a known length of time (number of black flies captured per 10 minutes). We also made qualitative collections of the black flies that swarmed about us, by sweeping an insect net over our heads.
Immature Stages
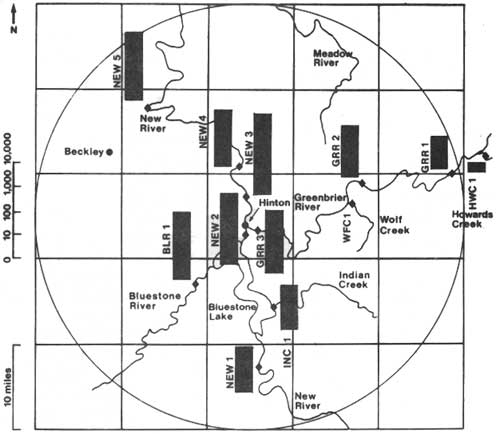
Previously published information on the suspected pest, Simulium jenningsi, indicated that this species prefers streams greater than 25 ft. wide (Underhill 1944, Snoddy and Noblett 1976, Stone and Snoddy 1969). Therefore, we sampled for the immature stages primarily in the New, Greenbrier, and Bluestone Rivers. There were 5 sites on the New River from Glen Lyn, VA to McCreery, WV, 3 sites on the Greenbrier River from Ronceverte to Willow Wood, and 1 site on the Bluestone River in the gorge at Pipestem (Fig. 2). In addition, many smaller streams, particularly those around Beckley. Pipestem, and Glade Springs, were also examined. Each site was first searched by qualitative means to determine if black flies were present. At those sites where black flies were present, quantitative bottom samples were taken with either a Surber Sampler or a Portable Invertebrate Box Sampler. Both devices are quantitative because they estimate the density of organisms per unit area of stream bottom (number of immature black flies per m2).
Life Cycles
Analyzing the life cycle of an aquatic insect involves determining the length of time that is required to pass from the egg to adult stages and the number of times that a species repeats this cycle (generations) in a year. Life cycles are analyzed by collecting immature stages at specified time intervals and then measuring the specimens with a microscope to determine their growth from one date to the next. We compared the life cycles of the black flies at 3 sites: New River below Bluestone Dam, Indian Creek, and Glade Creek. We collected immature stages with a fine mesh net (0.1 mm) at these sites every 2 weeks from April to October 1982.
Feeding Habits of Invertebrate Predators
In order to obtain preliminary information on the importance of black fly larvae in the food web of the New River, we studied the feeding habits of some aquatic insects that are known to be predaceous. These predators included: the hellgrammite, Corydalus cornutus, 2 net-spinning caddisflies, Hydropsyche hoffmani and Hydropsyche hageni, and a damselfly, Hetaerina americana. We collected these species at 3 sites on the New River (Bluestone Dam, Longbottom, Sandstone Falls) and then examined their gut contents by cutting them open under a microscope. We analyzed the importance of black flies in the food web by determining the relative abundance (volume) of black flies in the diet of the predators.
RESULTS AND DISCUSSION
Adults
A total of 6 species of black flies were collected by all means of adult sampling. In the quantitative samples, 99% of the organisms were Simulium jenningsi. The qualitative samples of black flies swarming about us were not enumerated but it appeared that most of those organisms were also S. jenningsi. The only exceptions occurred early and late in the period of adult emergence (April to October), when some of the swarms attracted to the investigators were composed primarily of S. vittatum.
While we were collecting samples we recorded some observations on the blood-feeding habits of the females. The investigators found biting by S. jenningsi to be exceptionally rare in relation to the numbers of black flies that would swarm about us at a given time. We often found ourselves surrounded by annoying swarms that were estimated to contain several thousand black flies, but we would be bitten only once or twice at most. Usually the investigators received no bites from black flies. Almost all of the black flies that were caught feeding on humans and horses were S. jenningsi. Early in the season (April 13, 1982) some Prosimulium spp. were caught feeding in the ears of a horse, and later in the season (June 10, 1982) one S. decorum was caught feeding on my arm. Coffman (1982) also reported S. decorum feeding on horses.
The data from the vehicle-mounted trap and carbon dioxide trap were analyzed by several standard statistical procedures (Voshell 1983a). There was a trend for the highest densities of adult black flies to occur along a NW-SE axis that approximately parallels the course of the New River (Fig. 1) It is interesting to note that two of the sites with consistently higher catches were located at Glade Springs Resort (Site 12) and Pipestem State Park (Site 17). Both of these places have been major sources of complaints about black flies. Detailed statistical analyses that compared the number of adult black flies with various parameters such as temperature, time, humidity, distance from Hinton, and wind, failed to explain the distribution patterns. The inability of the statistical analyses to explain the distribution of the adults probably indicates that dispersal is controlled by a complex interaction of many factors. The environs at Pipestem State Park and Glade Springs Resort have several features in common. Both are located on plateaus at the edge of steep-sided valleys through which New River tributaries flow (Bluestone River and Glade Creek, respectively), and both are developments where there are large open fields surrounded by woods. It is likely that the adults migrate along the streams (Wenk 1981), and the developed areas (particularly golf courses) provide ideal microenvironments for seeking hosts during the day and resting in trees at night (Wolfe and Peterson 1960).
Another observation that merits attention is that the second highest catch among the vehicle-mounted trap samples during one period was at Mountain Lake, VA (Site 25). The Mountain Lake site is over 25 miles from Hinton, yet it is only 5.5 miles from the closest stretch of the New River which lies in Virginia above Bluestone Lake. These results would seem to indicate that S. jenningsi might occur in the New River throughout the study area. However, the geographical extent of larval breeding can be determined conclusively by examining the results of the quantitative sampling in the streams and rivers.
Larval Breeding Sites
A total of 8 species were identified from samples of the immature stages. However, I will restrict my comments to S. jenningsi in order to keep this paper within a reasonable length. We examined 31 sites for the presence of immature black flies; S. jenningsi larvae were found at 12 sites (Fig. 2). The densities of the larvae at these 12 sites were analyzed by standard statistical procedures (Voshell 1983a) to determine the relative importance of different streams as breeding areas for S. jenningsi. Howard Creek and Wolf Creek had significantly lower densities of larvae than the other sites. The highest densities occurred in the New River just below Blueston Dam (NEW2) and 6 miles downstream at Longbottom (NEW3), however, statistical tests indicated that there were no significant differences among most of the sites on the New River, Greenbrier River, Bluestone River, and Indian Creek. To a statistician, this means that the same densities of larvae could be encountered in all of these streams. This information is very important for planning a program to manage the populations of black flies. High numbers of S. jenningsi larvae occur in many places in southern West Virginia, and most of these sites cannot be ignored, because there are no economic or nuisance thresholds to indicate what density of larvae can be expected to cause problems in the adult stage. The presence of S. jenningsi larvae at many sites also means that there is great potential for recolonization throughout southern West Virginia because the adults are known to disperse widely. Therefore, the larval breeding sites that produce significant numbers of adult S. jenningsi total approximately 130 miles of 4 different streams. This information is in sharp contrast to the speculation by Amrine (1982), who thought that S. jenningsi only occurred in significant numbers for a distance of 7 miles in the New River below Bluestone Dam. The differences in interpretation are probably the result of the qualitative methods used by Amrine (1982) and the quantitative methods used in this study.
|
| Fig. 1. Average catches (n=4) of adult black flies in the vehicle-mounted trap (number/1000m3) according to study sites during June 16-July 1, 1982. All data were transformed by √x. |
|
| Fig. 2. Average densities (n=3-5) of S. jenningsi larvae (number/m2) according to study sites during Sept. 7-15, 1982. All data were transformed by log10 (x + 1). |
Life Cycles
The life cycle of S. jenningsi was analyzed in the New River just below Bluestone Dam and in Indian Creek. S. jenningsi apparently overwinters as eggs which hatch in March. In the New River, the first generation emerged as adults during the last half of April. Four more generations followed, with the fifth generation emerging as adults around the first of October. All larvae completed their development and disappeared from the New River by late October. In Indian Creek, there was no indication of a generation completing its development prior to the middle of May. S. jenningsi only completed three generations in Indian Creek and disappeared from the stream by mid-September. The differences in the life cycle of the same species in two streams were probably due to different temperature regimes and available food in the streams (Anderson and Cummins 1979, Colbo and Porter 1979, 1981, Merritt et al. 1982). It is likely that there is a slightly different developmental pattern in each stream where S. jenningsi occurs. If an attempt is made to reduce the populations of S. jenningsi larvae throughout southern West Virginia, the application of the pesticide will probably have to be timed differently in each stream.
Role of Black Flies in New River Ecosystem
The environmental conditions and ecological relationships below a surface-release dam on a large, warm-water river (such as Bluestone Dam on New River) are very different from most free-flowing surface waters. A review of the downstream effects of impoundments can be found in a book edited by Ward and Stanford (1979). An impoundment with a surface-release dam introduces large quantities of living plankton and bacteria-rich detritus into the receiving stream. In response to this abundant supply of high quality food, there are very high densities of filter-feeding insects in the tailwaters below a dam. Filter feeders employ a variety of methods to "strain" or "sieve" seston (small particles of organic matter suspended in, the water). Readers should see a recent review by Wallace and Merrit (1980) for further information on filter feeding. In the New River just below Bluestone Dam, we found filter-feeding caddisflies and black flies to be very abundant (7,000/m2 and 9,200/m2, respectively). Another characteristic of ecosystems below surface-release dams is that there are relatively few species of insects because the temperature of the water is more consistent and warmer than an unregulated river. Many aquatic insect species require a fluctuating temperature cycle and cannot reproduce in tailwaters. In the New River, several species of caddisflies, black flies, and mayflies, and an assemblage of midge species accounted for over 87% of the densities of all organisms.
The species of aquatic insects that are suited to the environmental conditions below a surface-release dam often achieve very high levels of production because of the rich food supply. Production can be explained as the weight that organisms gain during a specified time interval. Fish also achieve high levels of production in tailwaters because of the bountiful supply of insects for them to eat, and the New River below Bluestone Dam is well known for its productive sport fishery. Therefore, tailwaters are very productive systems, but they are relatively simple systems. The high levels of production of the organisms are entirely dependent upon the high energy input from the upstream reservoir. The situation below dams is somewhat analogous to managed agricultural ecosystems, which depend on high inputs of energy to maximize the production of crops grown in monocultures. When simple systems are disturbed, the results are usually more pronounced than in complex systems which have more ways to compensate.
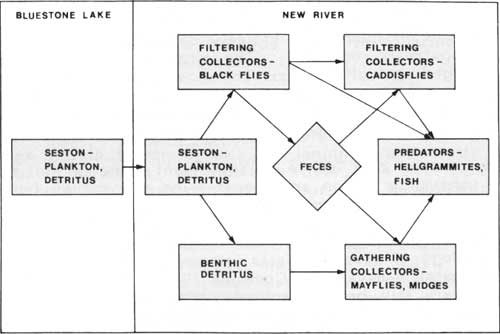
Black fly larvae may serve a significant function in flowing-water ecosystems by consuming seston and transferring the energy from the seston. In tailwaters, black flies could be especially significant because they are so abundant. In the past decade, aquatic ecologists have begun to integrate their knowledge of the feeding habits and physiology of aquatic insects into conceptual models of energy flow through flowing-water ecosystems (Cummins 1974), however, many details of energy transfers are still being resolved. With this caveat in mind, let us examine two ways that black fly larvae could function to transfer energy in the New River ecosystem. The most obvious way is that they can be consumed by invertebrate predators and fish (Fig. 3). Hence, the energy that black fly larvae assimilate from the seston would be transferred to higher trophic levels. In our preliminary studies (Kondratieff and Voshell 1983), we found that black flies comprised about 20% of the diet of insect predators at Bluestone Dam and 10% farther downstream at Longbottom and Sandstone Falls. These values indicate that black flies are not the single most abundant item in the diets of insect predators, but they are consumed in quantities that must be considered important, particularly in the area just below Bluestone Dam. Black flies may be especially important in the diets of hellgrammites, which consume black flies directly and also consume caddisflies that feed upon black flies. Hellgraminites are found in greatest numbers just below Bluestone Dam, and it is there that fishermen and commercial bait dealers go to collect them. The decrease in consumption of black flies downstream is consistent with the trend for decreasing densities of black flies downstream. Black flies exhibit their highest densities immediately below dams where the rich food is released and then progressively decrease in numbers downstream as the quality of the food diminishes.
Another way that black fly larvae could transfer energy in flowing water ecosystems is by producing feces which are consumed by other organisms (Fig. 3). Black flies only assimilate about 2% of the seston that they consume, and the remaining 98% is passed out of their bodies as feces. Black fly feces consist of particles which are larger than the seston particles that they originally consumed, therefore, black flies make food available to detritus-feeding organisms that do not have access to very fine particles suspended in the water. By combining information on ingestion and assimilation (Wotton 1978), area of habitat (Amrine 1982), and densities and life cycles (Voshell 1983a), I have calculated that black flies in the New River from Bluestone Dam to Sandstone Falls may produce 26 tons of feces per day for a total of 3,900 tons per year. This information certainly suggests that black fly larvae may be an integral component of the very productive New River ecosystem.
|
| Fig. 3. Conceptual model of energy flow from seston in Bluestone Lake to organisms in New River below dam. Developed from Cummins (1974) and Cummins and Klug (1979). |
In spite of this broad base of information that has been developed over the past several years, there are still several questions that need numerical answers to clear up the speculation. Some of the key points that require quantification are: (1) the production rate of black flies and other dominant aquatic insects; (2) the amount of insect predator production that is dependent on the function of black flies as prey; and (3) the amount of insect detritivore production that is dependent on the function of black fly feces for food. We currently have studies in progress that are designed to resolve each of these matters (Voshell 1983b). We are collecting monthly samples from the New River to quantify the production rates and trophic basis for the production of 2 species of black flies, 1 species of caddisfly, 2 species of mayflies, 1 type of midge, and the hellgrammite. The results will be the weights of these species that are produced in a year. By examining the gut contents of the predators, we will be able to determine how much of their weight is produced from eating black flies. The most challenging problem is to quantify the transfer of energy by means of black fly feces. Black fly feces are quickly dispersed in flowing water and cannot be recognized from other detritus. We are conducting laboratory studies in stream microcosms that will provide indirect information on this subject. By measuring the growth of selected detritus feeders in microcosms that only differ in the presence or absence of black flies, we will be able to determine the amount of production that is attributed to the consumption of black fly feces. The results of these studies will be available late in 1984.
SUMMARY
Simulium jenningsi is abundant throughout southern West Virginia because it breeds in many locations, has slightly different life cycles in these locations, and disperses widely. Before any attempts are made to manage the populations of S. jenningsi in the New River, the function of black flies in the unique ecosystem below Bluestone Dam should be thoroughly understood.
BIBLIOGRAPHY
Amrine, J. W. 1982. The New River connection in the black fly problem in southern West Virginia. W.V. Univ. Agric. Forestry Exper. Sta. 678: 1-30.
Anderson, N. H. and K. W. Cummins. 1979. Influences of diet on the life histories of aquatic insects. J. Fish. Res. Bd. Can. 36: 335-342.
Barnard, D. R. 1979. A vehicle-mounted insect trap. Can. Entomol. 111: 851-854.
Coffman, C. C. 1982. Investigation of black fly feeding on livestock in West Virginia and their vectoring of livestock parasites. Progress Report, Plant Pest Control Div., W.V. Dept. Agric.
Colbo, M. H. and G. N. Porter. 1979. Effects of food supply on the life history of Simuliidae (Diptera). Can. J. Zool. 57: 301-306.
Colbo, M. H., and G. N. Porter. 1981. The interaction of rearing temperature and food on the life history of two species of Simuliidae (Diptera). Can. J. Zool. 59: 158-163.
Colbo, M. H. and R. S. Wotton. 1981. Preimaginal blackfly bionomics. Pages 206-226 in M. Laird, ed. Blackflies. The future for biological control methods in integrated control. Academic Press, New York.
Cummins, K. W. 1974. Bioscience 24: 631-641. Structure and function of stream ecosystems.
Cummins, K. W. and M. J. Klug. 1979. Feeding ecology of stream invertebrates. Ann. Rev. Ecol. Syst. 10: 147-172.
Cupp, E. W. 1981. Blackfly physiology. Pages 199-206 in M. Laird, ed. Blackflies. The future for biological control methods in integrated control. Academic Press, New York.
Fallis, A. M., G. F. Bennett, G. Griggs, and T. Allen. 1967. Collecting Simulium venustum females in fan traps and on silhouettes with the aid carbon dioxide. Can. J. Zool. 45: 1011-1017.
Kondratieff, B. C. and J. R. Voshell, Jr. 1983. A study of the black fly invertebrate predators in the New River below Bluestone Dam. Report submitted to W. V. Dept. Nat. Resour. 42 pp.
Merritt, R. W., D. H. Ross, and G. J. Larson. 1982. Influence of stream temperature and seston on the growth and production of overwintering larval black flies (Diptera: Simuliidae). Ecology 63: 1322-1331.
Peterson, B. V. 1981. Simuliidae. Pages 355-391 in Manual of Nearctic Diptera, Vol 1. Res. Branch. Agric. Can. Monograph No. 27.
Snoddy, E. L. and K. L. Hays. 1966. A carbon dioxide trap for Simuliidae (Diptera). J. Econ. Entomol. 59: 242-243.
Snoddy, E. L. and R. Noblet. 1976. Identification of the immature black flies (Diptera: Simuliidae) of the southeastern U.S. with some aspects of the adult role in transmission of Leucocytozoon smithi to turkeys. S.C. Agric. Exper. Sta. Clemson Univ. Tech. Bull. 1057: 1-58.
Stone, A. and E. L. Snoddy. 1969. The black flies of Alabama (Diptera: Simuliidae). Auburn. Univ. Bull. 390: 1-93.
Underhill, G. W. 1944. Blackflies found feeding on turkeys in Virginia, (Simulium nigroparvum Twinn and Simulium slossonae Dyar and Shannon). Va. Agric. Exp. Sta. Tech. Bull. 94: 1-32.
Voshell, J. R., Jr. 1983a. Assessment of black fly populations in a portion of southern West Virginia. Report submitted to W. V. Dept. Nat. Resour. 134 pp.
Voshell, J. R., Jr. 1983b. Trophic basis of production for macroinvertebrates in the New River below Bluestone Dam. Research proposal submitted to W. V. Dept. Nat. Resour. 8 pp.
Wallace, J. B. and R. W. Merritt. 1980. Filter-feeding ecology of aquatic insects. Ann. Rev. Entomol. 25: 103-132.
Ward, J. V. and J. A. Stanford, eds. 1979. The ecology of regulated streams. Plenum Press, New York.
Wenk, P. 1981. Bionomics of adult blackflies. Pages 259-279 in M. Laird, ed. Blackflies. The future for biological control methods in integrated control. Academic Press, New York.
Wolfe, L. S. and D. G. Peterson. 1960. Diurnal behavior and biting habits of blackflies (Diptera: Simuliidae) in the forests of Quebec. Can. J. Zool. 38: 489-497.
Wotton, R. S. 1978. Growth, respirations, and assimilation of black fly larvae (Diptera: Simuliidae) in a lake-outlet in Finland. Oecologia 33: 279-290.
| <<< Previous | <<< Contents>>> | Next >>> |
newriver-84/sec20.htm
Last Updated: 08-Jul-2009