|
HOT SPRINGS
Analyses of the Waters of The Hot Springs of Arkansas Geological Sketch of Hot Springs, Arkansas |

|
THE CHEMICAL COMPOSITION OF THE WATERS OF THE HOT SPRINGS OF ARKANSAS.1
By J. K. HAYWOOD.
Chief of Miscellaneous Division, Bureau of Chemistry.
1Analyses performed at the Bureau of Chemistry, United States Department of Agriculture, under thed irection of H. W. Wiley, chief chemist.
INTRODUCTION
The Hot Springs of Arkansas are situated in Garland County immediately adjacent to Hot Springs City, on the western slope and at the base of Hot Springs Mountain, a spur of the Ozark Range. Originally there were said to have been 71 of these springs, but on account of improvements on the mountain, necessitating the merging of two or more springs into one, also by reason of the natural changes in the subterranean course of the water, this number has been reduced to 49. Forty-four of these are either in use or can easily be used by making some slight improvements. Five rise from the bed of the creek situated at the base of the mountain, and are consequently lost in the cold water of the stream. Besides the hot springs mentioned above, there are two cold springs in close juxtaposition on the northern slope of the mountain.
In making the analyses of these waters, because of changes apt to take place in certain constituents on standing, some of the determinations were made directly on the ground within one hour after the samples had been taken. The determinations mentioned are nitrogen, oxygen, carbon dioxide (free and as bicarbonates), nitrites, nitrates, oxygen consuming capacity, and free and albuminoid ammonia. Besides this, 10-gallon samples of each spring were shipped to Washington, D. C., where determinations of the various mineral constituents were at once begun. Each day the temperature of the spring then under analysis was taken; finally at the end of the chemist's stay at Hot Springs the temperatures were retaken in a single day, as well as the flow of each spring.
The constituents determined in each of the 44 hot springs and in the 2 cold springs include the following:
|
Oxygen, consuming capacity. Albuminoid ammonia. Free ammonia. Lithium. Sodium. Potassium. Magnesium. Calcium. Iron and aluminum. Manganese. Arsenic. Iodine. Bromine. |
Chlorine. Boric acid. Phosphoric acid. Nitric acid. Nitrous acid. Sulphuric acid. Silicic acid. Carbonic acid. Bicarbonic acid. Nitrogen. Oxygen. Hydrogen sulphide. Total solids. |
Besides these substances, the following were determined in spring No. 15 (Big Iron), which is not only the largest spring in the group but will serve as an example of all the other springs, since the chemical composition of all of them is so nearly alike:
Barium.
Strontium.
Fluorine.
In reporting the results of analysis, the bases and acids are given in parts per million of the positive and negative ions, except in the case of silica, which, in the present state of our knowledge, we can only report as such, not going into the question of how much is present as the silicic acid ion and how much present as free silica. Iron and aluminum are always reported together, because of the great difficulty in separating such small amounts of the two as appear in these waters. Wherever iron and aluminum are involved in any calculation the whole is considered as iron and given an atomic weight of 56. This is doubtless practically correct, since a test of the residue from a volume of one of the springs showed that the iron-aluminum precipitate consisted almost entirely of iron and contained aluminum, at the most, only in traces.
Because of the fact that these analyses will doubtless be referred to by many who have had no chemical training, the author has thought it best to combine the acids and bases in a hypothetical combination, thus reporting them as salts. That such a combination has no basis, in fact, is doubtless true, since we have every reason to believe that where various basic and acid ions are present in solution no base unites with any particular acid to the exclusion of all others, or vice versa, but that all possible combinations are formed, to at least some extent, of the various basic and acid ions present in solution. For example: Suppose we have calcium carbonate in solution. It partly dissociates into the positive and negative ions Ca and CO3 as follows:
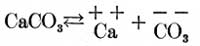
Again, if magnesium sulphate is in solution it partly dissociates as follows:
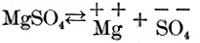
Now, if these two solutions are poured into each other, part of the calcium and sulphuric acid ions unite to form calcium sulphate, as follows:
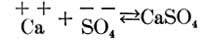
and part of the magnesium and carbonic acid ions unite to form magnesium carbonate, as follows:
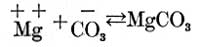
so that we have in solution not only the calcium carbonate, magnesium sulphate, and magnesium, calcium, carbonic acid, and sulphuric acid ions with which we started, but also some calcium sulphate and magnesium carbonate.
In calculating the above-mentioned hypothetical combination, sodium is joined to the nitrous and nitric acid ions; potassium to iodine and bromine; calcium to the phosphoric-acid ion and sodium to the metaboric-acid ion. Chlorine is assigned to the bases in the order NH4, Li, K, Na; sulphuric-acid ion in the order NH4, Li, K, Na Mg, Ca, and the residual bases are joined to bicarbonic-acid ion in the order Na, Mg, Ca, Mn, Fe. In case the bicarbonic-acid ion is not present in large enough amounts to join with all the remaining bases the residual calcium is joined to silica to form calcium silicate, and manganese and iron are calculated as Mn3O4 and Fe2O3, respectively.
| <<< Previous | <<< Contents>>> | Next >>> |
haywood-weed/intro1.htm
Last Updated: 22-Dec-2011