We dedicate this science summary to the memory of Les Viereck (1930-2008), fellow Alaskan botanist, ecologist and contributor of many superb herbarium specimens to the ALA herbarium.
Abstract
The Bering Land Bridge has been a major highway for Asian plants into North America, but also a barrier to some and a filter for others. Additionally, there are widely separated and highly local occurrences of Asiatic species in Alaska and adjacent Yukon that are thought by some to be relicts of late-glacial steppe-tundra. Molecular sequence data successfully clarify historical dispersal and vicariance events and will help resolve the role that Beringia played in facilitating and/or inhibiting plant migrations. Furthermore, hypothesis testing through coalescence-based analyses of molecular sequence data provides a powerful means of investigating the region’s floristic history.
Introduction
Nearly 80 years have passed since the term Beringia was coined by E. Hultén (1937) to refer to the immense unglaciated areas of Alaska and the adjacent regions of northwestern Canada and northeastern Russia. A vast literature on Beringia is now available that addresses questions of persistence in the glacial refugium, exchanges of plants between Asia and America, diversification of plants in the refugium, and Beringia as a source of tundra plants for post-glacial expansion into deglaciated areas beyond its borders.
Beringia as first proposed by Hultén has since been enlarged to encompass the entire region between the Lena River in northeast Russia, and the Mackenzie River in northwest Canada (Figure 1). At its closest point, North America is separated from Asia by only 50 miles (80 km), and portions of the strait are less than 100 feet (30 m) deep. Beringia was mostly exposed during the Tertiary, but the intercontinental land connection was flooded 4.8-5.5 million years ago (Marincovich and Gladenkov 2001). Throughout the Pleistocene, the sea level changed repeatedly, exposing and flooding the region
accordingly (Hopkins 1959). During the Last Glacial Maximum, the Bering Land Bridge was extant between 60,000 years ago and 25,000 years ago, and a sea-levels fell of as much as 397 ft (121 m) between 20,000-18,000 years ago, causing the maximum extent of the dry land connection between Asia and America during the Last Glacial Maximum. Only limited exchange was possible after about
11,000 years ago (Hoffecker et al. 1993).
It is quite impossible to understand fully the origin of Alaska’s flora without knowing a great deal about its Asian antecedents. During the Tertiary, easternmost Asia and northwestern North America were fully connected and essentially identical biotically. The Tertiary vegetation was dominated by mixed broad and needle-leafed trees. As climate cooled, forests receded from the region, but certain Tertiary floristic elements remained (Matthews and Ovenden 1990, Murray 1992).
The major contribution of Asian tundra plants ar-rived in Alaska throughout the Quaternary, via the land bridge while it was exposed. Successive changes directly related to the waxing and waning of glaciers and consequent rising and falling of sea level caused the repeated appearance and disappearance of the Bering Land Bridge. These dynamics have together created conditions for plant dispersal and also diversification. Even when the land bridge was submerged, plant propagules, driven by wind, are presumed to have crossed from Asia to America in winter when the Bering Strait was ice covered (Savile 1972).
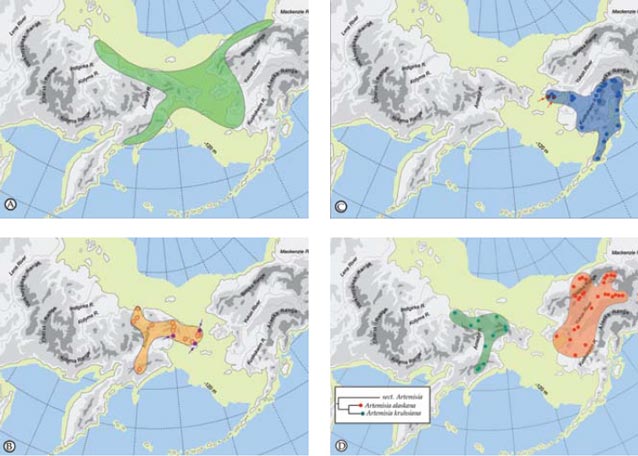
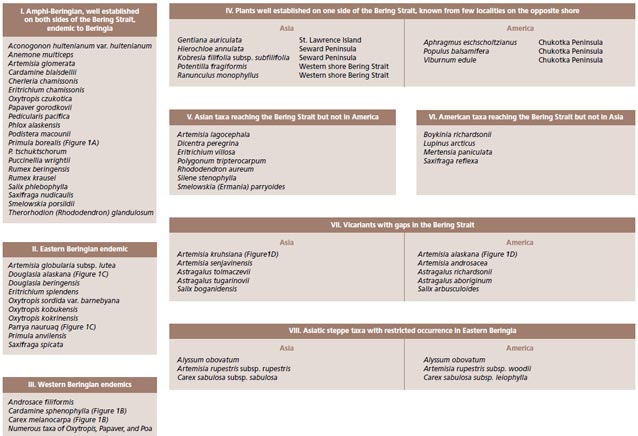
Whereas one might suspect that when the land bridge was exposed it provided relatively unimpeded dispersal routes between Asian and America, the distribution of plants shows that for some species the land bridge was a filter or even a complete barrier to movement. There are examples of some species that barely reach the opposite shore (Populus balsamifera and Viburnum edule on Chukotka and Potentilla fragiformis and Ranunculus monophyllus in Alaska) (Figure 2, pattern IV). A complex biogeographic history with distinct spatial and temporal components has created a flora rich in the circumpolar element but also one of vicariant taxa, intercontinental disjunctions and, for this latitude, a high level of endemism.
Several molecular studies support Hultén’s hypothesis that unglaciated Beringia was a Quaternary refugium for plants (Tremblay and Schoen 1999, Abbott et al. 2000, Thompson and Whitton 2006, Eidesen et al. 2007a,b). Beringia is therefore key to understanding post-glacial dynamics within and among species. Much of our understanding of Ice Age Beringia is based on the study of botanical specimens, and one of the largest collections for Alaska and adjacent areas is at the Herbarium (ALA) of the University of Alaska Museum of the North.

Dave Murray
Botanists in Alaska and adjacent Chukotka have long noticed a high degree of morphological and cytological diversification in well-established genera (Yurtsev 1999). We have now reached the limits of understanding diversification through traditional means, and we expect new molecular genetic evidence will shed light on the historical biogeography of Beringia.
Large scale biogeographic patterns observed in Beringia, aside from the fully circumpolar species, include taxa with an (a) amphiberingian distribution (taxa present on both sides of the Bering Strait and confined to the area between the Lena and Mackenzie rivers), (b) taxa restricted (endemic) to Western Beringia, (c) taxa that are endemic to eastern Beringia, and (d) taxa vicariant or with disjunct occurrences.
Reconstructing histories of taxa has long been an in-terest of botanists and requires the careful integration of phylogeny, biogeography, ecology and paleodistribution over time. The development of better analytical tools to infer historical biogeography as well as the ability to estimate dates for dispersal and diversification has set the stage for a new look at the origin and evolution of Beringia plants. This paper reviews the advances in studies of biogeographic patterns found in Beringia in the spirit of Eric Hultén’s visionary statement made in 1937: “…This land-mass, which I shall hereinafter call Beringia, must have been a good refugium for the biota during the glacial period...”.
Hypotheses and goals
Evidence from ecology, biology, geology, and biogeography has revealed complex patterns of diversification. It is now time to review these patterns, ask questions, and seek answers with molecular data as to the role Beringia played in shaping the composition of the flora today. We have developed four questions: (i) is there evidence for the Bering Land Bridge acting to structure genetic diversity? (ii) which factors allowed the Bering Land Bridge to act as a dispersal route for some plants, but not others? (iii) which plant groups show effects of a refugial existence in Beringia? (iv) are certain plant groups disjunct in Asia and America and recent arrivals to Beringia or are they relicts?
Materials and methods
Literature Review: Using Web of Science® we searched by keywords ‘Beringia’ and ‘plant’ (= 53 records) for an initial estimate of studies that have been conducted in Beringia. To focus the review further we surveyed the following journals for studies from 2003 (the last thorough review on the subject by Abbott and Brochman in 2003) to Dec. 2008: Systematic Botany, American Journal of Botany, Systematic Biology, Journal of Biogeography, Evolution, Molecular Ecology, and Science. We have limited our discussion to taxa that have a major distributional range within Beringia and have eliminated studies that are only marginal to the region.
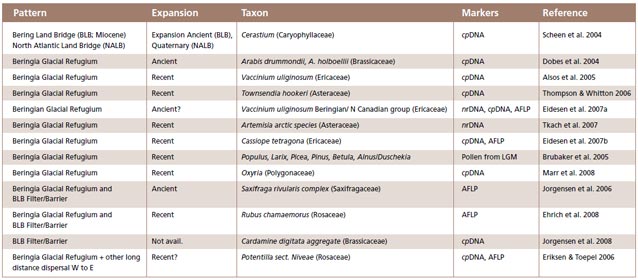
Evaluation of biogeographic patterns: The literature review revealed a large disparity in taxon sampling, molecular markers and general genetic patterns recorded. For each paper we recorded the type of material (mostly genetic marker) used and the general pattern of biogeo-graphic diversification reported, as well as whether diversification in the refugium was recent or ancient as a function of observed genetic diversity (Figure 4).
Results
New studies underscore the importance of Beringia in diversification of plants. Our survey of the literature from 2003 to 2008 identified 32 articles that involved ‘plants’ and ‘Beringia’. Overall, the majority of papers corroborates Hultén’s hypothesis of Beringia as an unglaciated Quaternary refugium, with some additional interesting findings relating to survival of certain boreal tree species in Beringia (Figure 4).
Beringia as a Glacial Refugium: Numerous studies have shown that Beringia acted as a refugium for arctic herbs and shrubs (Figure 4), but little is known about the role of this refugium for trees. A study by Brubaker et al. (2005) based on pollen and microfossils supports a glacial refugium for boreal Larix and Picea glauca in eastern Beringia, and Pinus pumila in Western Beringia. Similarly, Anderson et al. (2006) documented several populations with unique halpotypes and high allelic diversity in Alaska for Picea glauca, providing evidence that white spruce survived the Last Glacial Maximum in Eastern Beringia refugia, rather than having arrived by long-distance dispersal from areas outside of Beringia.
Bering Land Bridge– A Dispersal Highway: That the Bering Land Bridge has acted as a dispersal highway is supported by numerous studies and is evident in circumpolar plants studied thus far (Figures 2-3). In addition, species diversity with an amphiberingian distribution is very high (Figure 1A; Figure 2, pattern I), attesting to the evolution in Beringia due to glacial cycles. These dynamics have resulted in high levels of allopolyploidization and other evolutionary reticulations during speciation in Beringia tundra plants (Abbott and Brochmann 2003).

Rob Lipkin
Bering Land Bridge acting as a Filter: The Bering Land Bridge as a filter may be reflected in those taxa just managing a foothold on the opposite shore (Figure 2, pattern IV).
Bering Land Bridge acting as a Barrier: Although Western and Eastern Beringia share many species, more interesting are those taxa that are limited to either side of the Bering Strait (Figure 1B-C; Figure 2, patterns II, III). Other taxa are widespread throughout Asia and North America, reach the Bering Strait, but not the opposite side (Figure 2, patterns V-VI). We interpret these distributional patterns as evidence for the land bridge acting as a barrier to the “free” dispersal of taxa. Recently, Elias and Crocker (2008) documented a moisture barrier for the dispersal of steppe-tundra biota as an explanation for the disparate distribution of taxa common to Western Beringia but absent from Eastern Beringia.
Relicts or Recent Arrivals: While the geologic history of the Bering Land Bridge provides explanations for the complexity of today’s flora, the disjunct distribution of vicariant grasses and sagebrush (Artemisia) species of the same genera on either side of the Bering Strait are presumed related to relict tundra or steppes that were more wide-spread in the past (Murray et al. 1983) (Figure 2). In an exemplary study Alsos et al. (2007) document long-distance dispersal from several source regions to the arctic-archipelago Svalbard using DNA fingerprinting, and similar studies can now be undertaken for testing postglacial dispersal versus relictual distributions in Beringia.
For example the recent study by Tkach et al. (2007) shows that Artemisia kruhsiana from Western Beringia and Artemisia alaskana from Eastern Beringia are sister taxa based on analysis of molecular data in a large phylogeny of the genus Artemisia (Figure 1D; Figure 2, pattern VII). We intend to use a larger number of accessions throughout their biogeographic range to confirm whether these two morphologically very close taxa are indeed distinct. Subspecies rank for these taxa has recently been proposed by Elven and Murray (2008).
Future prospects
Explicit tests of the aforementioned hypotheses are needed to clarify the role that the changing Beringia environment played in the distribution of its flora. Molecular analyses at the intraspecific level, focused on the historical associations among populations, promise to reveal historic dispersal and vicariant events (Avise 2000). Because of the variation inherent in the evolutionary process among genetic loci, an approach for testing the fit of gene trees to models of population divergence is necessary. Genetic simulations based on coalescent theory (Kingman 2000) provide such tests by generating expected distributions of gene trees given population models. DeChaine (2008) demonstrated the utility of this approach for the Beringia flora, while underscoring the limited number of datasets available for such analyses and the demand for further analyses.
References
Abbott, R.J., L.C. Smith, R.I. Milne, R.M.M. Crawford, K. Wolff, and J. Balfour. 2000.
Molecular analysis of plant migration and refugia in the Arctic. Science 289: 1343-1346.
Abbott, R.J., and C. Brochmann. 2003.
History and evolution of the arctic flora: in the footsteps of Eric Hultén. Molecular Ecology 12: 299-313.
Alsos, I.G., T. Engelskjøn, L. Gielly, P. Taberlet, and C. Brochmann. 2005.
Impact of ice ages on circumpolar molecular diversity: insights from an ecological key species. Molecular Ecology 14: 2739-2753.
Alsos, I.G., P. Bronken, D.E. Eidesen, I. Skrede, K. Westergaard, G.H. Jacobsen, J.Y. Landvik, P. Taberlet, and C. Brochmann. 2007.
Frequent Long-distance plant colonization in the changing arctic. Science 316: 1606-1609.
Anderson, L.L., F.S. Hu, D.M. Nelson, R.J. Petit, and K.N. Paige. 2006.
Ice-age endurance: DNA evidence of a white spruce refugium in Alaska. Proceedings of the National Academy of Sciences (USA) 103: 12447-12450.
Avise, J.C. 2000.
Phylogeography: The History and Formation of Species. Harvard University Press. Cambridge, MA.
Brubaker, L.B., P.M. Anderson, M.E. Edwards, and A.V. Lozhkin. 2005.
Beringia as a glacial refugium for boreal trees and shrubs: new perspectives from mapped pollen data. Journal of Biogeography 32: 833-848.
DeChaine, E.G. 2008.
A bridge or a barrier? Beringia’s influence on the distribution and diversity of tundra plants. Plant Ecology and Diversity 1: 197-207.
Dobes, C.H., T. Mitchell-Olds, and M.A. Koch. 2004.
Extensive chloroplast haplotype variation indicates Pleistocene hybridization and radiation of North American Arabis drummondii, A. divaricarpa, and A. holboellii (Brassicaceae). Molecular Ecology 13:
349-370.
Ehrich D., I.G. Alsos, and C. Brochmann. 2008.
Where did the northern peatland species survive the dry glacials? Cloudberry (Rubus chamaemorus) as an example. Journal of Biogeography 35: 801-814.
Eidesen, P.B., I.G. Alsos, M. Popp, Ø. Stensrud, J. Suda, and C. Brochmann. 2007a.
Nuclear vs. plastid data: complex Pleistocene history of a circumpolar key species. Molecular Ecology 16: 3902-3925.
Eidesen, P.B., T. Carlsen, U. Molau, and C. Brochmann. 2007b.
Repeatedly out of Beringia: Cassiope tetragona embraces the Arctic. Journal of Biogeography 34: 1559-1574.
Elias, S.A., and B. Crocker. 2008.
The Bering Land Bridge: a moisture barrier to the dispersal of steppe-tundra biota? Quaternary Science Reviews 27: 2473-2483
Elven, R., and D. F. Murray. 2008.
New combinations in the panarctic vascular plant flora. Journal of the Botanical Research Institute of Texas 2: 433-446.
Eriksen, B., and M.H. Töpel. 2006.
Molecular phylogeography and hybridization in members of the circumpolar Potentilla sect. Niveae (Rosaceae). American Journal of Botany 93: 460-469.
Guggisberg, A., G. Mansion, S. Kelso, and E. Conti. 2006.
Evolution of biogeographic patterns, ploidy levels, and breeding systems in a diploid-polyploid species complex of Primula. New Phytologist 171: 617-632.
Hoffecker, J.F., W.R. Powers, and T. Goebel. 1993.
The colonization of Beringia and the peopling of the world. Science 259: 46-53.
Hopkins, D.M. 1959.
Cenozoic History of the Bering Land Bridge. Science 129: 1519-1528.
Hultén, E. 1937.
Outline of the history of arctic and boreal biota during the Quaternary Period. Bokfölags Aktiebolaget Thule. Stockholm, Sweden.
Jørgensen, M.H., R. Elven, A. Tribsch, T.M. Gabrielsen, B. Stedje, and C. Brochmann. 2006.
Taxonomy and evolutionary relationships in the Saxifraga rivularis complex. Systematic Botany 31: 702-729.
Jørgensen, M.H., T. Carlsen, I. Skrede, and R. Elven. 2008.
Microsatellites resolve the taxonomy of the polyploidy Cardamine digitata aggregate (Brassicaceae). Taxon 57: 882-892.
Kingman, F.C. 2000.
Origins of the Coalescent. Genetics 156: 1461-1463.
Marincovich, L., and A.Y. Gladenkov. 2001.
New evidence for the age of Bering Strait. Quaternary Science Reviews 20: 329-335.
Marr, K.L., G.A. Allen, and R.J. Hebda. 2008.
Refugia in the Cordilleran ice sheet of western North America: chloroplast DNA diversity in the Arctic-alpinge Oxyria digyna. Journal of Biogeography 35: 1323-1334.
Matthews, J.W., Jr, and L.E. Ovenden. 1990.
Late Tertiary Plant Macrofossils from Localities in Arctic/Subarctic North America: A Review of the Data. Arctic 43: 364-392.
Murray, D.F., B.M. Murray, B.A. Yurtsev, and R. Howenstein. 1983.
Biogeographic significance of steppe vegetation in subarctic Alaska. Permafrost: Forth International Conference Proceedings. 883-888.
Murray, D.F. 1992.
Vascular plant diversity in Alaskan arctic tundra. The Northwest Environmental Journal 8: 29-52
Petrovsky, V.V. 1975.
Cardamine. In Flora Arctica URSS, Vol. 7, edited by A.I. Tolmatchev. Nauka, Leningrad
Savile, D.B.O. 1972.
Arctic adaptations in plants. Monograph No. 6, Canada Department of Agriculture. Ottawa, Canada.
Scheen, A.C., C. Brochmann, A.K. Brysting, R. Elven, A. Morris, D.E. Soltis, P.S. Soltis, and V.A. Albert. 2004.
Northern hemisphere biogeography of Cerastium (Caryophyllaceae): insights from phylogenetic analysis of noncoding plastid nucleotide sequences. American Journal of Botany 91: 943-952.
Thompson, S.L., and J. Whitton. 2006.
Patterns of recurrent evolution and geographic parthenogenesis within apomictic Easter daisies (Townsendia hookeri). Molecular Ecology 15: 3389-3400.
Tkach, N., M.H. Hoffmann, M. Roeser, A.A. Korobkov, and K.B. von Hagen. 2007.
Parallel evolutionary patterns in multiple lineages of arctic Artemisia L. (Asteraceae). Evolution 62: 184-198.
Tremblay N.O., and D.J. Schoen. 1999.
Molecular phylogeography of Dryas integrifolia: glacial refugia and postglacial recolonization. Molecular Ecology 8: 1187-1198
Yurtsev, B.A. 1999.
Survey of Arctic Legumes with emphasis on the species concept in Oxytropis. In The species concept in the High North- A Panartic Flora Initiative, edited by I. Nordal and V. Razzhivin, 295-318. The Norwegian Academy of Science and Letters. Oslo, Norway.
Part of a series of articles titled Alaska Park Science: Volume 8, Issue 2: Park Science in the Arctic.
Last updated: August 10, 2016
